Bryophytes and lichens are an important component of biodiversity (Rozzi et al. 2008) and play a key role in ecosystems such as peatlands (Minayeva 2008). Some of them, specifically Sphagnum spp., have been considered ecosystem engineers. These organisms directly or indirectly modulate the availability of resources to other species and, additionally, they modify, maintain and/or create habitats (Jones et al. 1994). Moreover, the ground layer in peatlands is dominated by a 90-100 % cover of bryophytes (Vitt & Belland 1995) and the functions of the peatland ecosystem is highly dependent on this layer. For instance, nutrient sequestration, water-holding abilities, decomposition, and acidification are all influenced by this layer (Vitt 2000). Nevertheless, these cryptogamic groups are rarely included in floristic and ecological studies (Pharo et al. 1999, Lang et al. 2009). Southern South America is no exception.
Vast expanses of peatland can be found in Chilean Patagonia. A significant number of peatlands were formed by peat accumulation in open water after glacial retreat (Heusser 1984, Villagrán 1988, Villagrán 1991), referred to here as glaciogenic peatlands (GP). However, in northern Patagonia, the use of fire and clearcutting since the middle of the 19th century in places with low drainage have created areas of wetlands dominated by species of the genus Sphagnum L. (Zegers et al. 2006, Díaz et al. 2008). When Tepualia forests (TF), characterized by poor drainage, are burned or cleared, waterlogged conditions hinder forest recolonization and stimulate Sphagnum colonization (Díaz et al. 2007, Díaz & Silva 2012). These habitats are called anthropogenic peatlands (AP).
Besides, TF are swamp habitats, closely related to peatlands. This type of forest is dominated by Tepualia stipularis (Hook. & Arn.) Griseb. (Myrtaceae), and can be associated with Podocarpus nubigenus Lindl. (Podocarpaceae), Pilgerodendron uviferum Florin. (Cupressaceae) and/or Drimys winteri J. R. Forst. & G. Forst. (Winteraceae). It grows in waterlogged areas (García & Ormazabal 2008) and accumulates organic matter (Veblen & Schlegel 1982). Several studies show that peatlands and TF have been linked by their floras demonstrating that the vascular and bryophytic floras of these habitats are highly similar (Villagrán & Barrera 2002, Villagrán et al. 2002, Villagrán et al. 2003, Villagrán et al.2005). Díaz et al. (2008) reported differences in floristic composition that allow to distinguish between GP (natural) and AP. However, there are no comprehensive studies that quantify and compare the floristic composition of AP, GP, and TF. Another connection is that these ecosystems are seriously threatened. Peatlands are threatened and degraded because peat extraction and Sphagnum harvesting, mainly to use as a substrate in horticulture (Díaz & Silva 2012). In Chile, Sphagnum exports increased by over 400 % between 2002 and 2011 (ODEPA 2016). TF are also threatened because their firewood is one of the main energy sources on the island (Neira & Bertin 2010).
We are studying AP as an ecosystem that has been shaped by human activity. Its transformation of landscape has caused changes to biological communities, posing new challenges for traditional thinking in conservation and resource management (Lindenmayer et al. 2008). Taking into account this changes in ecosystem-human relation, Milton (2003) presented a novel concept of emerging ecosystems. This concept defines an ecosystem whose species composition and relative abundance have not previously occurred within a given biome. The key characteristics of these ecosystems are: new species combinations, with the potential for changes in ecosystem functioning, and they are the result of deliberate or inadvertent human action, but do not depend on continued human intervention for their maintenance (Hobbs et al. 2006). Under this concept we wonder, if AP is a novel or an emerging ecosystem?
In this research, we study alpha and beta diversity of mosses, liverworts, and lichens in AP, GP, and TF of Isla Grande de Chiloé (Chile). In particular, we address the following questions: i) Are there significant variations in species composition between the studied habitat types? ii) Are AP more floristically related to TF? iii) Do GP (natural habitats) have a higher bryo-lichenic diversity than AP? iv) Is there evidence to recognize AP as a novel ecosystem?
Methods
Study Site. The study area was located in the Isla Grande de Chiloé, Los Lagos Region, Chile (42°-43° S and 73°-75° W). The prevailing climate is wet temperate with a strong oceanic influence (di Castri & Hajek 1976). The total annual rainfall is about 2,300 mm (CONAF 2009), reaching 5,000-6,000 mm in some areas, with a mean summer temperature of 10.2 ºC and a mean winter temperature of 6.2 ºC (Pérez et al. 2003).
We selected ten sites located in the northern and central parts of the island (Figure 1). Two kinds of Sphagnum peatlands were studied, which were defined according to their origin and their characteristic vegetation (Díaz et al. 2008). Three study sites represented the glaciogenic peatland type (GP): Río Negro (GP-RN), Los Caulles (GP-CA) and Púlpito (GP-PL); five study sites represented the anthropogenic peatland type (AP): Senda Darwin (AP-SD), Lecam (AP-LC), Pumanzano (AP-PM), Río Chepu (AP-CH) and Teguel (AP-TG). In addition, two sites represented the Tepualia forest type (TF): Chiloé National Park (TF-CU) and another area of Senda Darwin (TF-SDB) (Figure 1).

Figure 1 Location of studied site in Chiloé. Glaciogenic peatlands (GP) (black circles): PL, Púlpito; CA, Caulles; and RN, Rio Negro. Anthropogenic peatlands (AP) (gray circles): SD, Senda Darwin; CH, Chepu; PM, Pumanzano; LC, Lecam and TG, Teguel. Tepualia forests (TF) (white circles): CU, Chiloé National Park and SDB, Senda Darwin forest.
Species composition. On each site we established three lineal transects of 50 m. In each transect, three equidistant sample plots were placed. We extracted a block from the surface layer measuring 20 × 20 × 10 cm from each sample plot. These blocks were used to evaluate species richness and biomass, following Bullock’s harvest method (1997). Dry biomass was used to estimate species abundance for each sample plot. Specimens were carefully determined according to morphological characters, and their characteristics were compared with the literature (Engel 1978, Schuster 2000, Schuster 2002, Larraín 2007, Buck & Goffinet 2010), type specimens or other herbarium specimens deposited in PC, S, MACB and CONC herbaria. For lichens, chemical characters were also used. Lichen substances were identified using thin layer chromatography (TLC), following the protocol of White & James (1985). Specimens were deposited in MACB and CONC herbaria.
Although Drosera uniflora Willd. is a vascular plant, its presence was registered due to its great significance as an indicator of GP species. Due to their tiny size, the liverworts Calypogeia sphagnicola, Cephalozia skottsbergii and Hyalolepidozia bicuspidata were considered as a functional group. Likewise, the lichens Cladonia pycnoclada, C. mitis and C. arbuscula subsp. squarrosa were considered as the subgenus Cladina following Ruoss and Ahti (1989), because chemical tests are required for correct determination. Appendix 1 includes a list with the collected species.
Data analysis. Alpha diversity was evaluated in two scales following Gray’s (2000) concepts: point species richness (SRP), the species richness of a single sampling unit (quadrant); and sample species richness (SRS), the species richness of a number of sampling units from a site of a defined area (site). In addition, we calculated the Shannon diversity index (H’) and evenness (J’) to combine the effects of species richness and abundance (Magurran 2004). To assess changes in species composition among habitat types, we calculated beta diversity using the Bray-Curtis dissimilarity index (Bray & Curtis 1957). Moreover, cluster analysis was performed using the unweighted pair group metric with averaging method (UPGMA) and Bray-Curtis presence/absence distance to evaluate the resemblance among sites. Non-metric multidimensional scaling (NMDS) was used to compare plant communities from AP, GP, and TF. Relative abundance and Bray-Curtis distance was used as a general measure of ecological similarity for NMDS ordination (Beilman 2001). Analysis of similarities (ANOSIM) was used to test for differences in species composition for the three habitat types. R values of ANOSIM were generated using 9,999 random permutations. We used the Non-Parametric Kruskal-Wallis H ANOVA to test significant differences in the richness and diversity measures among habitats and sites.
We employed PAST (Hammer et al. 2001) for indices, cluster analysis, NMDS and ANOSIM. STATISTICA 7.0 (StatSoft 2004) for the Kruskal-Wallis H test.
Results
Alpha Diversity. A total of 86 species was found: 42 liverworts, 29 mosses, 14 lichens and one insectivorous flowering plant. Fifty three-point five percent (53.5 %) of the species were found at only one site and are here considered potentially rare within the studied peatlands: 16 mosses, 21 liverworts and 9 lichens. AP had a total of 52 species (18 mosses, 21 liverworts and 13 lichens), TF had 45 species (15 mosses, 29 liverworts and 1 lichen), and GP had 21 species (4 mosses, 13 liverworts and 3 lichens) (Figure 2). Of the 86 species, 29 were only found in TF, 27 were exclusive of the AP and five occurred only in GP. Nevertheless, we found shared species between habitat types, nine species between AP and GP, nine species between AP and TF, six species between TF and GP, and seven species that were shared among the three habitats.

Figure 2 Species richness of mosses, liverworts, and lichens, in the three habitat types. Anthropogenic peatlands (AP), glaciogenic peatlands (GP) and Tepualia forests (TF).
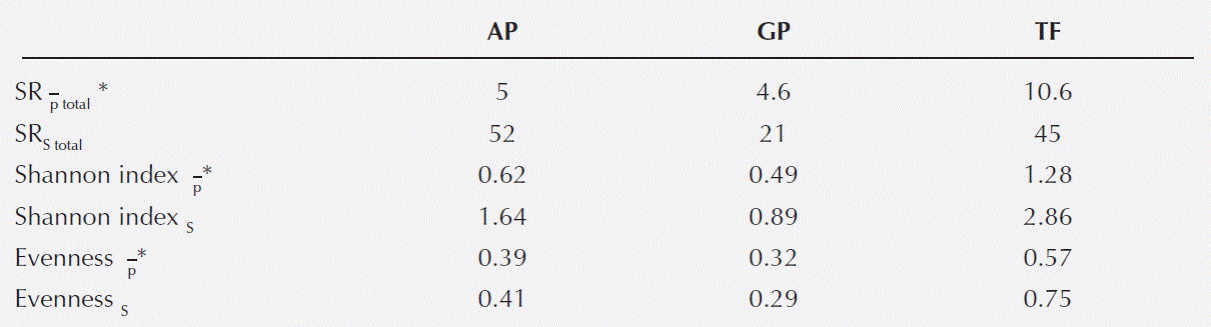
SRP, SRS and diversity indices were significantly different among the three habitat types. TF showed the highest SRP and diversity index, while AP presented the highest SRS (Table 1).
Table 1 Species richness and diversity index by habitat type. SRP, point species richness; SRS, sample species richness;
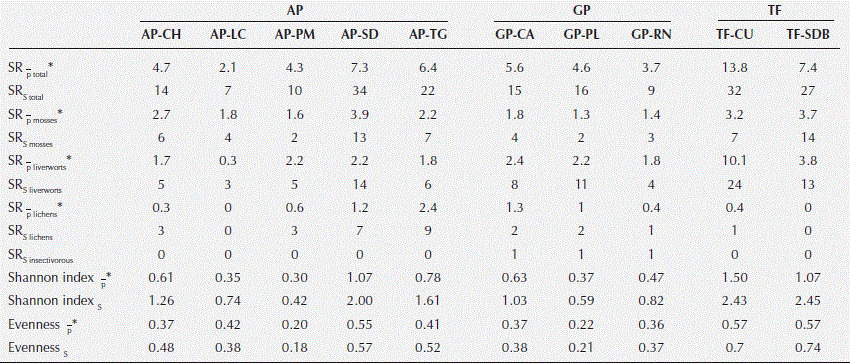
Species richness and abundance were significantly different between study sites (Figure 2 y 3). SRS ranged between 7 and 34 species where AP-SD and TF-CU were the highest, and AP-LC and GP-RN the lowest. SRP ranged between 2 and 14 species per quadrant. Diversity indices followed the same trends in species richness where AP-SD, TF-SDB and TF-CU had the highest values. Nevertheless, AP-PM and AP-PL presented the lowest diversity indices.

Figure 3 Relative species abundance of mosses, liverworts, and lichens in the three habitat types. Anthropogenic peatlands (AP), glaciogenic peatlands (GP) and Tepualia forests (TF).
When analyzing the SRS per botanical groups, it was seen that AP-SD and TF-SDB had the highest number of mosses, TF-CU the most liverworts and AP-TG and AP-SD the most lichens.
Beta Diversity. Dendrogram of floristic composition based on Bray-Curtis similarity clearly shows two groups of habitats with a similarity of over 30 % (Figure 4). The first group included three locations, TF-SDB and TF-CU (both TF, which had a similarity of 48 %, and AP-SD, which is a sister site of the clade formed by these sites. The second group included the seven remaining locations. In this group, AP-LC and AP-TG were individually separated and sub-grouped with a similarity of 45 %. Within this sub-group, two subclusters were formed: GP-RN, GP-PL and GP-CA, which have a similarity of 60%, and AP-PM and AP-CH with a similarity of 58 %.

Figure 4 Bray-Curtis similarity dendrogram of floristic composition among studied habitats. Tepualia forests (+): TF-CU, Chiloé National Park and TF-SDB, Senda Darwin forest. Anthropogenic peatlands (*): AP-CH, Chepu; AP-LC, Lecam AP-PM, Pumanzano; AP-SD, Senda Darwin; and AP-TG, Teguel. Glaciogenic peatlands (**): GP-CA, Caulles; GP-PL, Púlpito; and GP-RN, Rio Negro.
Based on non-metric multidimensional scaling (NMDS), the structure in species composition revealed differences in the habitat type (Figure 5). In this plot we can see a clear separation of TF samples (white circles), while AP (black diamonds) are arranged in a dispersed form in the plot and not distantly separated from GP (grey hexagons). AP and GP samples are closer than TF.
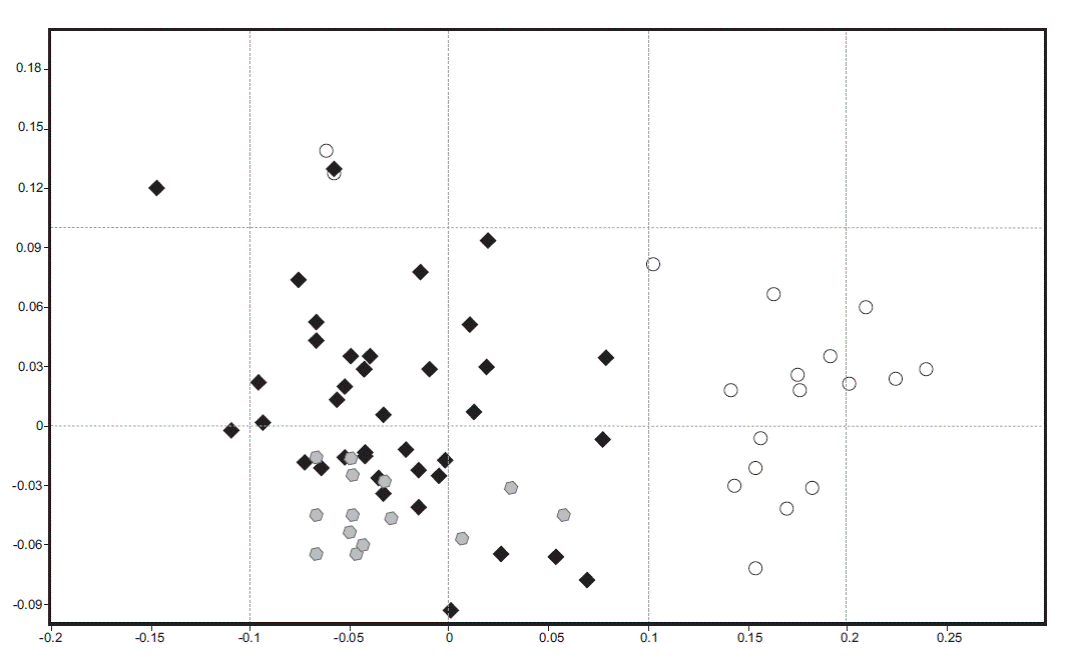
Figure 5 Non-parametric multidimensional scaling ordination representing similarity in bryophyte and lichen species composition between habitat types. Black diamonds represent anthropogenic peatlands (AP), grey hexagons glaciogenic peatlands (GP), and white circles Tepualia forests (TF) (n = 90).
The statistical significance of differences in abundance and species composition among habitats was confirmed by the ANOSIM tests (R ANOSIM global = 0.412, p = 0.0001). When ANOSIM pair-wise comparisons were performed, we detected that TF vs. AP (p = 0.0001; R ANOSIM = 0.770) and GP vs. TF (p = 0.0001; R ANOSIM = 0.930) were significantly different in species composition; however, AP vs. GP (p = 0.3453; R ANOSIM = 0.012) were more closely related.
Discussion
Cryptogamic diversity. Bryophyte and lichen diversity have repeatedly been underestimated due to limited knowledge of these groups, especially in southern South America. Indeed, our research demonstrates the importance of these groups as our results show that the total bryophyte and lichen diversity were considerably higher than that previously reported from Patagonian peatlands. Díaz et al. (2008) reported 27 species of bryophytes and lichens in peatlands of Chiloé, Villagra et al. (2009) recorded five species of terricolous lichens in Sphagnum peatlands of Aisén, and Kleinebecker et al. (2010) found 54 bryo-lichenic species in the peatlands of Magallanes. In our study, 56 species of bryophytes and lichens were reported for peatlands of Chiloé (AP and GP). Under these circumstances, in a diversity context, the peatlands of Los Lagos Region are as rich as the peatlands of Magallanes, which have been considered significantly important due to their location. The Magallanes Region reports the highest diversity of bryophytes and lichens in the country (Goffinet et al. 2006). On the other hand, if the number of species recorded in this study is compared with species found in bogs of the Northern Hemisphere, for instance, Canada with 36 species (Vitt & Belland 1995) or Britain with 39 species (Wheeler 1993), the diversity observed in our study shows great relevance. This is especially remarkable because the peatlands of the Southern Hemisphere are under-represented compared to the vast percentage of land they occupy in the Northern Hemisphere (Joosten & Clarke 2002).
Differences between habitats. Our results also showed differences in species richness, composition and diversity indices among study sites and among types of studied habitats (Table 1). This trend is more evident in the AP group, as seen in the cluster analysis (Figure 4). This analysis shows that GP and TF form delimited groups and have less within-group variability, while AP are more heterogeneous. These differences could be related to processes carried out in the cryptogamic community establishment, and the chemical and topographic characteristics of the sites. For instance, AP-SD has greater similarity to TF and this similarity could be attributed to its early stage of formation. In this locality, the peat layer was the smallest of the sampled sites and is located very close to the forest. Moreover, it could be labeled as an ecotonal zone between the forest and peatland. Another highlighted example is AP-LC, which is part of the AP and GP cluster; however, it is the first to diverge from the group. This site has particular hydrological characteristics (León et al. In review) because the water level is very high and not significantly lower in summer; something that does not happen in any of the other study sites. Furthermore, the highest richness, diversity index and number of exclusive species were observed in AP. In contrast, GP showed the lowest values of these community parameters. TF showed a high richness, independent of the scale (point or sample), and this habitat type presented a large number of exclusive species. These results can be understood by their phytogeographic location. TF form part of the temperate forest of Chile, an ecosystem that has been classified as a biodiversity hotspot for conservation of global significance by its uniqueness and high threats (Myers et al. 2000). Moreover, in NMDS (Figure 5) and ANOSIM analyses, we could also see clear differences among the three habitat types. There were significant differences in the floristic composition of GP, AP, and TF, where GP and AP were more closely related to each other because they share a large number of species and environmental characteristics. On the contrary, TF were distantly related to the other two groups. The significant differences between AP and GP in floristic composition concurred with Díaz et al. (2008) who described differences in floristic composition between GP (natural) and AP. Nonetheless, the clear differences between the flora of peatlands and TF reported in this research differs from previously published studies (Villagrán & Barrera 2002; Villagrán et al.2002; Villagrán et al. 2003; Villagrán et al. 2005) that suggest that the flora of peatlands and TF are similar.
Table 2 Species richness and diversity index by sites. SRP, point species richness; SRS, sample species richness;
Novel ecosystems, ecosystem services and implication for management. According to our results, AP have very distinct and singular characteristics. They are characterized by high values of diversity (Table 1), with a large number of endemic species of southern South America (León et al. 2014). Moreover, new records for the bryophyte flora of the island and Chile have been found (León et al. 2013). In addition, even when all species growing in AP belong to the Valdivian ecoregion and it is not possible to attribute them to other biomes, these ecosystems show a singular composition of species that did not previously occur when they were TF (before clearcutting). These new species combinations have the potential to change ecosystem functions, as discussed below. They are the result of human action, but do not depend on continued human intervention for their maintenance. According to Hobbs et al. (2006), these are all characteristics that define a novel ecosystem. Therefore, applying the concepts of Hobbs et al. (2006) and Milton (2003), we can denominate AP as a novel ecosystem. In these novel ecosystems, the new species compositions have deeply changed the landscape of the island and ecosystem services. Díaz & Armesto (2007) showed that Sphagnum cushions could act as a nursery species, facilitating the establishment of Embothrium coccineum. Nevertheless, these cushions could inhibit the establishment of pioneer species such as Drimys winteri and Baccharis patagonica in successional scrubs of Chiloé, which is a limiting factor for forest regeneration. On the other hand, the colonization and the establishment of large populations of Sphagnum in sites where the forest was removed have also changed ecosystem functioning. León & Oliván (2014) found that AP are accumulating peat and therefore are also acting as carbon sinks and reservoirs of freshwater; ecosystem services relevant to the island. It is important to highlight that the peatlands of Chiloé are threatened and degraded by Sphagnum harvesting, especially AP. Unfortunately, Chile has no legal regulation for the extraction of Sphagnum moss. These sites have been excessively exploited without sustainable protocols, and as a consequence, they show evident signs of overexploitation. This imposes the need to promote conservation and restoration of these ecosystems. However, three important questions arise and require a deeper analysis: What would be the direction of the restoration in AP? Would it be to recover the temperate rainforest (historical setting)? Or would it be to recover telmatic wetlands formed after a disturbance? These are significant points of discussion about the conservation and management of these emerging ecosystems, because in many parts of the world, primary motivations for ecosystem management are associated with human survival rather than considerations of historic fidelity (Hobbs et al. 2009). In this case, considering their high social and economic value, a focus on ecosystem functions rather than recomposition of species (historical) or the cosmetics of landscape surfaces would be useful according to Choi (2007). A reasonable way would be to promote the growth of Sphagnum and to restore the ability to store water and peat. This would have a significant impact on local communities because Chiloé peatlands are very important for fresh water supply on the island. This island has no freshwater input from snowmelt as found on mainland Chile; the freshwater input is mainly from precipitation (Zegers et al. 2006). Thus, according to the climate change scenario, low rainfall rates in recent years means that freshwater on the island is at risk and the conservation of peatlands is of even more importance to the population.
Finally, we do not know about the dynamics of species composition under new abiotic conditions, especially Sphagnum species, which are ecosystem engineers. Therefore, it is necessary to increase efforts to understand their functioning and the main environmental factors driving these ecosystems. Moreover, these studies could provide insights into the effects that global change factors can have on these novel ecosystems, and could provide important information for management and ecological restoration.











 nueva página del texto (beta)
nueva página del texto (beta)


