The Mexicali Valley is located in northeastern Baja California, south of the Imperial Valley in California. Approximately 70 % of the cultivated land in Mexicali is irrigated by gravity flow with water from the Colorado River (Daesslé et al., 2009). In recent years the Mexicali industrial unit such as piping steel, paint making, agriculture, paper mill, fish cultivation, electroplating industries drain their wastewater into the river (Garcia-Hernandez et al., 2006). As a result of these emissions and the fact that metals are non-biodegradable, heavy metals accumulate in soils affecting the environment, leading to a potential toxic exposure of the population (Wong et al., 2006). In recent years, a variety of techniques have been employed to clean- up the soils and effluents. These methods include ion exchange, chemical precipitation, and disinfection, adsorption by activated carbons, reverse osmosis and nanofiltration (Machado et al., 2010). However, these methods are expensive, require high energy and are not able to completely remove the heavy metals. In contrast phytoremediation is proposed as a cost effective alternative for the treatment of contaminated soils (Alkorta et al., 2004). Many plant species are able to grow under heavy metals polluted environments (González-Mendoza and Zapata-Perez, 2008).
The genus Prosopis L. is characteristic of arid and semiarid zones, and its widespread distribution includes ecosystems in Asia, Africa, and the Americas (Pérez-García et al., 2012). The species of this genus are characterized by high individual genetic variability at the population level, which influences the responsiveness of these species to extrinsic variables, such as climate, drought, alkalinity and salinity (Shiferaw et al., 2004). In Mexico, the majority of the populations of the genus Prosopis are found in the northern and central of the country, where they have formed forest extensions that are adapted to desert climate.
Species such as Prosopis glandulosa Torr., P. juliflora (Sw.) DC., P. pubescens Benth. and P. palmeri S. Watson dominate the vegetation mosaic in Baja California Mexico, primarily in an ecosystem known as mezquitales, which is characterized by a hyperarid climate with almost no rainfall (0.6 mm year-1) (Carevic, 2014). These populations of the genus Prosopis have been the focus of scientific interest mainly because of their physiological and ecological adaptations to their hyperarid environment. However, little research has analyzed the current physiological tolerance to heavy metals of P. juliflora. The few previous studies of P. juliflora have focused mainly on the evaluation of ability of these plants to germinate in different copper concentrations (Victor et al., 2007). However, previous studies using P. juliflora foliar disc have demonstrated that the uptake of copper in excess by plants can initiate a disorder of numerous physiological functions causing damage at the cellular level (Michel-López et al., 2014). Therefore a better understanding of the strategies that are used by individuals of P. juliflora for copper tolerance will help researchers to develop biotechnological alternatives as the phytoremediation in northwest of Mexico. In this context, the main objective of this research was evaluated the physiological responses of P. juliflora to copper an essential element. This assessment was accompanied by analyzing the influence of different concentrations of copper on the photosynthetic status and bioaccumulation of this metal in P. juliflora plants in a short time. We presume that individuals of P. juliflora denote a high accumulation of copper in response to stress caused by acute exposition to copper.
Materials and methods
Germination of Prosopis juliflora. The seeds were donate for the National Forestry Commission of Mexico, in Mexicali, B.C. Seeds were surface-sterilized by soaking in 1 % NaOCl (Clorox) for 5 min and finally rinsed with deionized sterile water four times altogether (for ensuring pathogen-free seed) and dried at room temperature. After disinfection, the seeds were germinated in sterilized sand (grain size 6 to 9 mm), in a greenhouse under the following conditions: temperature range 30 to 34 ºC during the day, 28 to 30 ºC during the night, 12 h light: dark photoperiods and 60 % relative air humidity. Once the seedlings developed a few roots, they were transplanted to single pots (0.5 L) containing a commercial potting soil mix combined with quartz sand and peat moss (5% soil, 20 % sand and 30 % peat moss) previously sterilized in hot water (100 ºC), during 2 h. The plants were irrigated daily with water and every other week, fertilized with Hoagland solution without additional Cu2+.
Exposure to Cu 2+. Twenty two-months old plants showing similar leaf number and size were randomly allocated (n = 5) and transferred to individual plastic containers holding 500 mL of deionized water solution with or without 10, 50 and 100 mM of copper sulfate (CuSO4) at pH: 5.5 for 48 h under hydroponic conditions. After 48 h plants were uprooted from the plastic containers and aerial parts (leaves and stems) and roots were collected for further analysis. These exposures were performed in quadruplicates.
Copper analysis. To remove residual copper solution, root samples were washed for 5 min in dideionized water, then for 10 min in 20 mM EDTA, and again rinsed for 5 min in dideionized water. Five gram (fresh weight) of leaves, stem, and roots was dried for 72 h at 70 °C. Sample of dried tissue (500 mg) was digested in 10 mL of nitric acid (85 % v/v) overnight according to González-Mendoza et al. (2007).
Resulting digests were diluted up to 10 mL with dideionized water and Cu2+ concentration was determined for each sample by an inductively coupled plasma optical emission spectrophotometer (ICP-OES 400 Perkin-Elmer USA) using standards of 50, 20, 5, 0.5 mg L-1 of Cu2+ (Sigma Chem., St. Louis, MO, USA) for calibration curves and readings were evaluated at 324.8 nm absorbance against 1 % HNO3 (Estrella-Gómez et al., 2009). The concentration of Cu2+ in plant tissues was expressed in μg g-1 on a dry weight (dw) basis.
Bioaccumulation factor and translocation factor. The index of the ability of the plant to accumulate copper with respect to its concentration in the medium, bioaccumulation factor (BAF), was calculated according to Ghosh and Singh, (2005). The relative translocation of copper from roots to other parts of the plants, the translocation index (Ti), was calculated according to Singh et al. (2011).
Chlorophyll fluorescence measurement. The chlorophyll fluorescence was measured using a Plant Efficiency Analyser (PEA, Hansatech Instruments Ltd., King’s Lynn Norfolk PE32 1JL, UK) on completely expanded leaves. The leaves were subjected to a 5 min period of adaptation to darkness under to induce the complete oxidation of the reaction centers using light exclusion clips (González-Mendoza et al., 2013).
The maximum photochemical efficiency of photosystem II (Fv/Fm), the activity of photosystem II (F v /F 0 ) and the efficiency of the water splitting apparatus represent by (Fo/Fv) were measurements of five plants for each treatment, at 8, 12, 24, and 48 h after exposure to copper levels using a leaf one per plant analyzed. The plants exposed to the copper were arranged in a complete randomized design, with four replications and five plants per plot.
Statistical analysis. Data were analyzed with analyses of variance (ANOVA), and mean were comparison test (Tukey´s α = 0.05) was performed (Statistical Package version 5.5, Statsoft, USA). Significant differences were accepted if P < 0.05 and data was expressed as mean ± Standard error.
Results
Cu 2+ concentration in different tissues of Prosopis juliflora. In the present study P. juliflora plants grew well in the presence of 10, 50 and 100 mM Cu+2, and no showed physiological changes (eg., discoloration of leaves) associated at heavy metal exposure. However, the Cu2+ accumulation on roots and aerial parts of P. juliflora varied with the different concentrations of copper sulphate solutions used in this experiment (Figure 1). In this sense 10, 50 and 100 mM Cu2+ doses caused significant changes in Cu2+ accumulation on roots and aerial parts compared with control roots. For example, the accumulation of Cu+2 in roots plants treated with 50 mM Cu2+ (0.522 μg g-1) did not show significantly change with plants treated with 100 mM Cu2+ (0.531 μg g-1) after 48 h of exposure. However, in case of aerial parts, the exposure at 10, 50 and 100 mM Cu2+ showed a gradual accumulation of copper in P. juliflora with respect to doses used. In this regard, the figure 1 show that the ranges of Cu+2 accumulation were 0.026, 0.158 and 0.433 μg g-1 in aerial parts, exposed to 10, 50 and 100 mM, respectively after exposure.
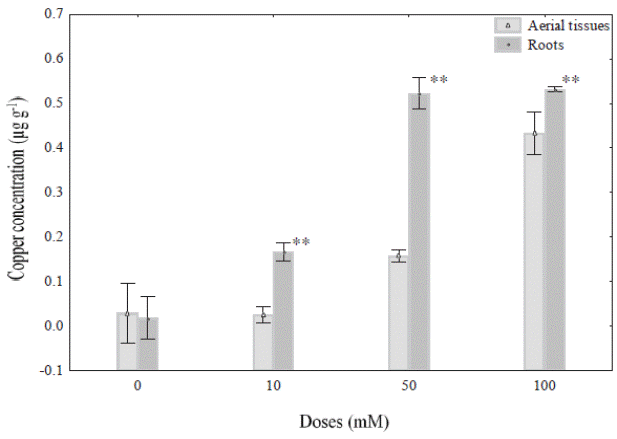
Figure 1 Copper concentration (μg g-1) in aerial parts and roots of Prosopis juliflora plants exposed to different concentrations (0,10, 50, and 100 mM) under hydroponic conditions during 48 h. Values are mean ± SD (n = 4). Significant differences among treatments are indicated by: * P < 0.05; ** P < 0.01.
Bioaccumulation factor and translocation index of Cu +2. Bioaccumulation factor (BAF) and translocation index (Ti) was significantly higher in root than that of aerial parts (stem and leaves) (P < 0.05; Table 1). However, its BAF was significantly less in roots of plant grown to 100 mM (66.41 ± 0.28) with respect to 10 (208.33 ± 9.01) and 50 mM (130.66 ± 3.54), respectively (Table 1).
Table 1 Bioaccumulation Factor (BAF) and Transportation Index (Ti) of copper in Prosopis juliflora (SW) during 48 h exposure.
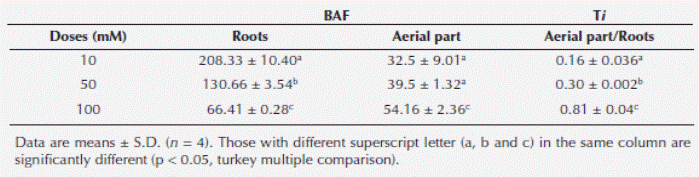
However, the aerial parts/roots Ti for plants exposed to 10 and 50 mM Cu+2 (0.16 ± 0.036; 0.306 ± 0.002, respectively) was very low, compared with the value showed by 100 mM Cu2+ (0.81 ± 0.04; Table 1).
Chlorophyll fluorescence measurement. The biophysical analysis of the leaf blades demonstrated that the size and number of active reaction center (Fv/Fo) and the maximum photochemical efficiency of photosystem II (Fv/Fm) showed a progressive drop with the greatest differences (relative to the control) being observed at the highest metal concentration at 24 h after exposure to metal (Figure 2A, C). On the other hand, the water splitting apparatus represented by (Fo/Fv) was significantly higher only in plants treated with 100 mM Cu+2 during the 24 and 48 h after metal exposure (Figure 2B).
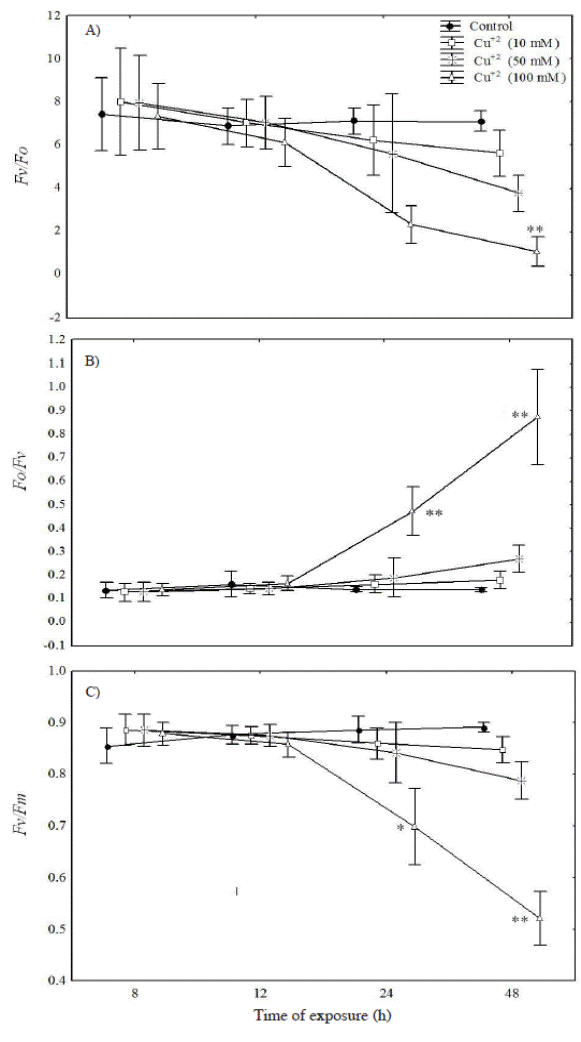
Figure 2 Fluctuations of various fluorescent parameters for Prosopis juliflora after 48 h exposure to 10, 50 and 100 mM Cu+2: A) the activity of photosystem II (Fv/F0); B) water splitting apparatus of PSII (Fo/Fv), and C) the maximal photochemical yield of PSII in dark adapted leaves (Fv/Fm). Significant differences among treatments are indicated by: * P < 0.05; ** P < 0.01.
Discussion
In order to maintain the absorption of essential metals within physiological limits and minimize their negative effects, many mesquite species have evolved a complex network of homeostatic mechanisms in roots that serve to control the uptake, accumulation, trafficking and detoxification of metals (Usha et al., 2009; Zappala et al., 2013). In this sense, our result revealed that the amount of copper accumulated in the roots of Prosopis juliflora indicated that this species have the ability to take up copper from the solution and to accumulate the metal in their roots, so they could be used to phytostabilize metal-polluted groundwater (rhizofiltration). This result might be related to the presence of exclusion and sequestering processes that moderate metal uptake by roots and induce their accumulation in tissues (González-Mendoza et al., 2007). The presence of higher values of Cu2+ found in roots and the low value for aerial parts/root translocation index found in P. juliflora exposure during 48 h, suggest a metal exclusion mechanism might be operating, as described by Baker (1981) and Boularbah et al. (2006).
In this context, Prosopis juliflora could not be considered as hyperaccumulator in view of less accumulation of Cu in roots and aerial parts. Because has lower concentrations of Cu+2 (0.531 μg g-1) than 1000 μg g-1, which are the limits prescribed for a hyperaccumulator (Boularbah et al., 2006; Zappala et al., 2014).
For evaluation of Prosopis juliflora plants as potential phytoremediators, low-cost, easy to carry and high throughput techniques are essential. In this sense, chlorophyll a flourescence represent an excellent screening tool for evaluation of heavy metal stress in aerial parts of plants (Kummerová et al., 2010; Hussain et al., 2011). In the present study the changes observed in Fv/Fm, Fv/Fo and Fo/Fv (chlorophyll fluorescence parameters) as result of exposition with Cu+2 demonstrated a rapid inactivation of photosystem II (PSII) in P. juliflora leaves. The fluctuations in these parameters can be the result of Ca+2 substitution by Cu+2 in the catalytic center of PSII during photoactivation. Another possibility is that Mn+2 is replaced by Cu+2 from the water-splitting apparatus at the oxidizing side, resulting in a disruption of photosynthetic reactions (Küpper et al., 2009). Additionally, the decline in the F v /F 0 ratio is indicative of a decline in the rate of photochemistry as the primary electron acceptor pool (Q n ) became increasingly oxidized, or a reduction of the pool size of the primary electron acceptors associated with PSII activity (Krause and Weiss, 1991). It may indicate that Fv/Fo and Fo/Fv parameters are the most sensitive components in the photosynthetic electron transport chain in the presence of high copper concentration. In agreement to these results, it has been previously confirmed that the Fv/Fo and Fo/Fv parameter are the most sensitive component of PSII under undesirable conditions (González-Mendoza et al., 2013). Similar results were observed by and Singh et al. (2013) who reported that higher concentrations of heavy metals induced one rapid inactivation of PSII in Luffa acutangula under hydroponics conditions.
Therefore the information provided by this short-term experiment in Prosopis juliflora showed that several physiological processes are activated, in which the copper uptake by roots and their accumulation in tissues play a central role. In this sense during short-term exposure to copper (48 h), sublethal effects such as alteration of the photosystem II, can be observed. It can thus be concluded that chlorophyll fluorescence parameters are sensitive as biomarkers of short-term copper exposures.
Conclusion
The results presented in this research demonstrate that exposure of Prosopis juliflora plants to excess Cu+2 causes specific negative effects on the photosystem II. Such alteration may have critical physiological implications under conditions of Cu+2 toxicity in this mesquite species. Finally, the present study showed that P. juliflora is a promising prospect for heavy metals phytoremediation purposes occurring in arid and semi-arid climates in the northwest Mexico.











 nova página do texto(beta)
nova página do texto(beta)


