Most environments vary in time; living species must be adapted to such environmental variations (Rees, 1994). Plant strategies to defer germination are expected to be more common in arid and semiarid environments with unpredictable suitable seasons for germination and seedling establishment in agreement with Jurado and Moles (2003). Gutterman (1993) says due to the short time span of suitable conditions for germination in arid zones, seeds must make the most of available moisture and germinate at high speed.
The uptake of water is triphasic with a rapid initial uptake (phase I, i.e. imbibition) followed by a plateau phase (phase II). A further increase in water uptake (phase III) occurs as the embryo axis elongates and breaks through the covering layers to complete germination (Finch-Savage and Leubner-Metzger, 2006). Imbibition is an essential process initiating seed germination. It is the first key event that moves the seed from a dry, quiescent, dormant organism to a resumption of embryo growth. Within the first 10 min of imbibition, the seed coat is wetted and adsorbed gases are released, immediately followed by an increase in respiratory activity; membrane reorganization, mitochondrial development, and associated increases in enzyme activity (McDonald, 1994). Upon imbibition, the quiescent dry seed rapidly resumes metabolic activity (phase II) (Bewley, 1997). Radicle extension through the structures surrounding the embryo is the event that terminates germination (phase III) and marks the commencement of seedling growth. This final phase has an increased water uptake driving cell expansion leading to the completion of germination. Induction of phase III can involve water channel proteins such as Intrinsic Proteins (PIPs) and Tonoplast Intrinsic Proteins (TIPs) that regulate the passage of water across membranes (Nonogaki et al., 2010).
Seeds are likely to remain viable after dehydration during the first two phases (Taylor et al., 1992). Some species have been detected to have more seed germination after a wetting and drying (HD) than after constant moisture in agreement with Vincent and Cavers (1978). For other species, germination percentage increases with increasing number of HD cycles, like in the study by Ren and Tao (2003) for Calligonum junceum and C. leucocladum, implying a cumulative effect of early imbibition (Hou et al., 1999). Seeds of some species seem to have “hydration memory” or a capacity to retain some of the physiological changes, like the differential expression of proteins (López-Urrutia et al., 2014), induced by imbibition even after temporary drying, having these seeds the same moisture content as the seeds that have never been hydrated (Dubrovsky, 1996).
For some species, seeds germinate faster after HD when compared to constant moisture (Idris and Aslam, 1975; Baskin and Baskin, 1982; Bradford et al., 1993; Fujikura et al., 1993). A fast germination can be advantageous in arid environments where the soil surface is unlikely to retain moisture for long periods (Meyer and Monsen, 1992; Flores and Jurado, 1998). However not all species have the same traits in similar environments (Budelsky and Galatowitsch, 1999). While the role of faster and higher seed germination after wet and dry cycles is likely to be a key factor in desert plant dynamics not many species have been tested (McDonough, 1964; Dubrovsky, 1996; 1998; Wilson and Witkowski, 1998; Huang and Gutterman, 2000; Tobe et al., 2001; Ren and Tao, 2003; Sánchez-Soto et al., 2005; Rito et al., 2009; Santini and Martorell, 2013; López-Urrutia et al., 2014). In here we determined if germination rate and germination percentage varied in relation with wet and dry cycles for nine Chihuahuan Desert species. We hypothesized that the seeds of desert species here studied can tolerate periods of dehydration after single or multiple hydration events and subsequently germinate more and faster.
Materials and methods
In September 2012, seeds from at least three mother plants for each species: Atriplex canescens (Pursh) Nutt. (Chenopodiaceae), Cucurbita foetidissima Kunth (Cucurbitaceae), Echinocactus platyacanthus Link & Otto (Cactaceae), Ferocactus pilosus (Galeotti ex Salm-Dyck) Werderm (Cactaceae), Lepidium virginicum L. (Brassicaceae), Lesquerella berlandieri S.Watson (Brassicaceae), Nassella tenuissima (Trin.) Barkworth (Poaceae), Sartwellia mexicana A.Gray (Asteraceae), and Yucca filifera Chabaud (Asparagaceae), were collected in the South of the Chihuahuan Desert (23° 36’ 43” - 25° 13’ 51” N and 100° 02’ 56” to 101° 17’ 28” W) between 1,800 and 2,000 m above sea level. The collected seeds were kept at room temperature in dry plastic jars for five months before the germination experiments. The vegetation in the region is a mixture of open low shrubland with abundant Muhlenbergia villiflora Hitchc. var. villiflora, Scleropogon brevifolius Phil., Bouteloua dactyloides (Nutt.) Columbus, Dasyochloa pulchella (Kunth) Willd. ex Rydb., Frankenia gypsophila I. M. Johnst., Dalea radicans S. Watson, Dalea gypsophila Barneby, Machaeranthera heterophylla L. R. Hartm., Gaillardia comosa A. Gray, Machaeranthera crutchfieldii B. L. Turner, Dicranocarpus parviflorus A. Gray, Frankenia margaritae Medrano, Isocoma gypsophila B. L. Turner, Aster gypsophila B. L. Turner, Nama hispida A. Gray var. gypsicola I. M. Johnst., Sartwellia mexicana, Strotheria gypsophila B. L. Turner, and Thelesperma scabridulum S. F. Blake (Estrada-Castillón et al., 2010).
Hydration and dehydration measurements. A randomized sample of ten seeds from a lot of around 1,000 seeds taken from not less than 30 fruits and three mother plants per species was weighed dry, then seeds were set with 25 ml of distilled water in a Petri Dish and seeds were weighed hourly until constant weight which was considered the maximum imbibition or stage 2 of water kinetics in seed germination. Seeds were then set to dry at room temperature and were weighed hourly until the initial dry weight was obtained. Maximum imbibition and drying times of seeds were used to define HD cycles in order to prevent seeds germinating during treatments.
Treatments. Prior to germination, 50 seeds by species (5 Petri dishes × 10 seeds each) were subjected to the following treatments of hydration/dehydration (HD): (1) One HD cycle (1 cycle = 24 h hydration and 5 d dehydration, (2) Three consecutive HD cycles, (3) A 72 h hydration and 5 d of drying, and (4) A control group to which no previous hydration was applied. Seed hydration, dehydration and germination were carried out in a Seedburo® seed germination chamber. Seed hydration-dehydration were carried out at 26 °C, the mean annual temperature from the study site in agreement with CONAGUA (2012), in open containers using 200 ml distilled water in January and February 2013.
Seeds were set to germinate inside Petri dishes using agar (16 %) as a constant moisture source at 30 °C with white led light for 12 h followed by a 12 h dark period at 16 °C from the 4th of February to the 4th of March 2013. For each species ten seeds were placed in each of five Petri dishes. Germination was measured daily for 30 d and was determined after protrusion of the radicle. Germination rate (t 50 ) was determined as the day in which at least 50 % of the germinated seeds was reached according to Jurado and Westoby (1992). Germination time was classified following Jurado and Westoby (1992) into: fast when 50 % of germinated seeds was reached by day 3, medium when 50 % germinated seeds was reached between days 4 and 6, and slow when 50 % of the germinated seeds was reached after day 6.
Statistical Analyses. When obtained data did not have a normal distribution these were transformed to arcsine in the case of percentage data (Sokal and Rohlf, 1995) and to the square root + 1 for t 50 (Sokal and Rohlf 1995). A one way ANOVA (P ≤ 0.05) and Tukey or Games-Howell tests were performed to find differences, using SPSS Statistics® 18. The factors to be analyzed were the four HD treatments and the dependent variables were germination percentage and germination rate (t 50 ).
Results
Imbibition and drying time. The kinetics of water uptake had a typical three phase profile (Figure 1 and Figure 2), due to scale differences in seed mass, two graphs were used, one for small-seeded species and one for large-seeded species. Smaller seeds averaging 0.001 g (± 0.0007 std) reached maximum imbibition (stage II) within 8 h. Stage 3 was reached within 72 h except for Lesquerella berlandieri that did not germinate. Dehydration occurred within 24 hours for all the small seeds (Figure 1).

Figure 1 Water absorption phases in seeds weighing 0.001 g ± 0.0007 std average. Seed mass was determined by weighing ten dry seeds for each species. Initiation of: ▲ phase I, ■ phase II, ◆ phase III, Kinetics of water uptake (continuous line), dehydra tion (discontinuous line)

Figure 2 Water absorption phases in seeds weighing 0.029 g ± 0.022 std average. Seed mass was determined by weighing ten dry seeds for each species. Initiation of: ▲ phase I, ■ phase II, ◆ phase III, Kinetics of water uptake (continuous line), dehydration (discontinuous line)
Maximum imbibition for species with larger seeds (average weight = 0.029 g ± 0.022 std) was similar to that of the smaller seeds (within 8 h) except for seeds of Yucca filifera that took 20 h (Figure 2). Dehydration occurred between 24 and 35 h.
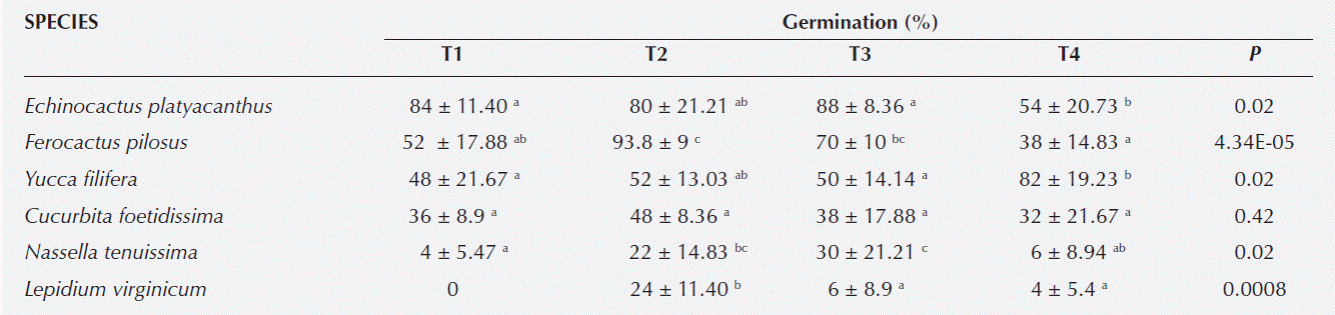
Germination percentage. From the nine species studied only Lesquerella berlandieri did not germinate. Atriplex canescens, Cucurbita foetidissima, and Sartwellia mexicana had similar germination across treatments. The highest germination percentages in Ferocactus pilosus (F = 16.08, d.f. = 3, P = 4.3E-05) and Lepidium virginicum (F = 9.42, d.f. = 3, P = 0.001) were observed in the treatment of three cycles, for Nassella tenuissima (F = 4.06, d.f. = 3, P = 0.02) the highest percentages were registered in the long cycle treatment. More seeds of Echinocactus platyacanthus (F = 4.36, d.f. = 3, P = 0.02) germinated with any of the HD treatments than with the control. Yucca filifera (F = 4.27, d.f. = 3, P = 0.02) seeds germinated more with the three cycles treatment and with the control (Table 1).
Table 1 Seed germination (%) ± standard deviation of species from Southern Chihuahuan Desert after four treatments of hydration/ dehydration (HD): T1) One HD cycle (1 cycle = 24 h hydration and 5 d dehydration, T2) Three consecutive HD cycles, T3) A 72 h hydration and 5 d of drying, and T4) A control group to which no previous hydration was applied. Different letters between rows indicate significant differences.
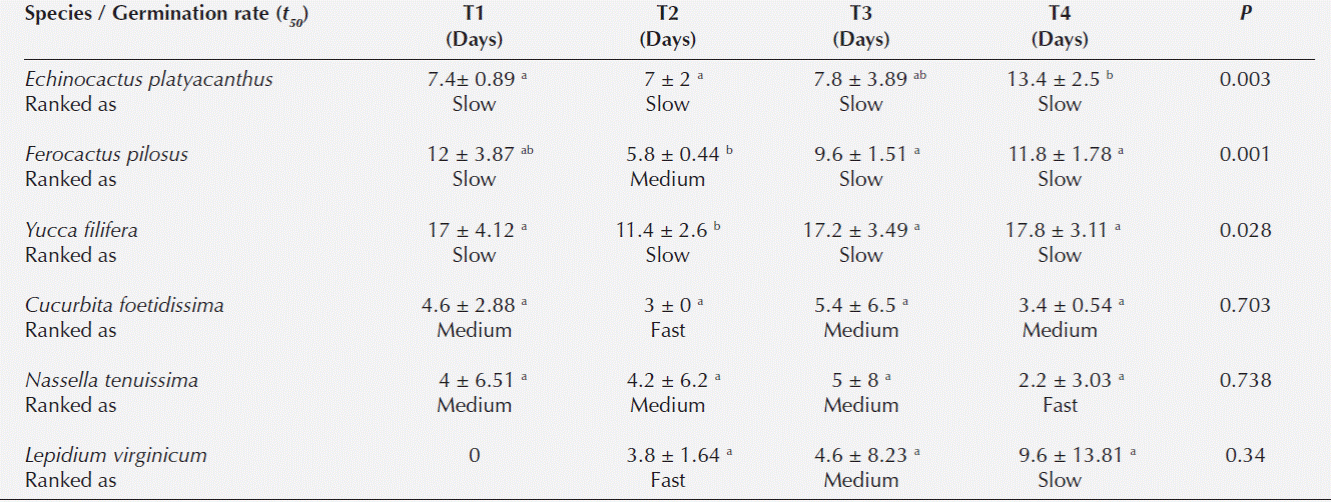
Germination rate (t 50 ). This variable was only analyzed for species with germination in at least one treatment higher than 15 % (Cucurbita foetidissima, Echinocactus platyacanthus, Ferocactus pilosus, Lepidium virginicum, Nassella tenuissima, and Yucca filifera) (Table 2). Treatments did not affect germination rate for N. tenuissima, L. virginicum and C. foetidissima (F = 0.42, d.f. = 3, P = 0.738; F = 1.19, d.f. = 3, P = 0.343; and F = 0.47, d.f. = 3, P = 0.703). Echinocactus platyacanthus seeds germinated faster with one short cycle and the three-cycle treatment (F = 6.92, d.f. = 3, P = 0.003). Seeds of F. pilosus and Y. filifera germinated faster with the three-cycle treatment (F = 8.01, d.f. = 3, P = 0.001; F = 3.9, d.f. = 3, P = 0.028) (Table 2). Germination time was intermediate and slow for most species across treatments (Table 2).
Table 2 Germination rate (t50) ± standard deviation of species from Southern Chihuahuan Desert after four treatments of hydration/dehydration (HD): T1) One HD cycle (1 cycle = 24 h hydration and 5 d dehydration, T2): three consecutive HD cycles, T3): A 72 h hydration and 5 d of drying, and T4): a control group to which no previous hydration was applied. Different letters between rows indicate significant differences.
Discussion
The three stages of water kinetic were clearly identified and this allowed us to determine the duration of the hydration/dehydration cycles for the species studied, such as has been determined for other cacti and legumes (Bewley and Black, 1985; Dubrovsky, 1996).
Water uptake by the seeds is influenced by permeability of the testa, environmental factors, seed shape and moisture content (Vertucci, 1989; Taylor et al., 1992). In this study some seeds completed imbibition within an hour, while for others it took up to 20 h perhaps as a result of differences in thickness of the seed coat or lower seed permeability. Imbibition times are likely to be longer in natural conditions depending on substrate and moisture availability (Tao et al., 2000).
Seeds can tolerate desiccation during stages 1 and 2 of the three water kinetic stages, but are intolerant during stage 3, hence hydration/dehydration treatments should be completed before germination (Taylor et al., 1992).
Zhu et al. (2014) say hydration/dehydration treatments can promote, inhibit or have no effect on seed germination. In our study only four species were affected by the treatments. From the eight species that had seeds germinating, four of them (Echinocactus platyacanthus, Ferocactus pilosus, Lesquerella virginicum, and Nassella tenuissima) showed higher germination percentages in one or more of the hydration/dehydration treatments than in the control group. The treatment of three cycles and the longer cycle treatment promoted the seed germination for four and two species, respectively, which is in agreement with Dubrovsky (1996) who found that repeated cycles of hydration/dehydration cause cumulative effect. It is possible that this process of hydration memory is related with the different protein expression as found for Ferocactus peninsulae (López-Urrutia et al., 2014).
For Yucca filifera germination was similar or higher in the control group, suggesting that hydration/dehydration does not promote germination for the species; however, for this species, t 50 was faster for seeds treated with the three-cycle treatment than for control.
Short rainfall may have accumulative physiological effects on seeds and this can be simulated using hydration/dehydration cycles to promote seed germination (Dubrovsky, 1996; Sánchez-Soto et al., 2005; Rito et al., 2009; Santini and Martorell, 2013; López-Urrutia et al., 2014). For seeds of Echinocactus platyacanthus, Ferocactus pilosus, and Yucca filifera the three hydration/dehydration treatment resulted in the shortest t 50 (6 d less than the control group). This faster germination time has been shown elsewhere for the cacti Carnegiea gigantea (McDonough, 1964), Stenocereus thurberi (McDonough, 1964; Dubrovsky, 1996; Sánchez-Soto et al., 2005), S. gummosus (Dubrovsky, 1998), S. alamosensis (Sánchez-Soto et al., 2005), Cereus jamacaru (Rito et al., 2009), Mammillaria hernandezii (Santini and Martorell, 2013), and F. peninsulae (Dubrovsky, 1996; 1998; López-Urrutia et al., 2014). Seed germination in arid lands is likely to be fast due to the short periods that the soil retains moisture (Jurado and Westoby, 1992; Gutterman, 1993). For Central Australia for instance, Jurado and Westoby (1992) found more than half of 105 species studied to germinate within 3 days of contact with water. In our study, however only three species germinated within 3 days of contact with water, and this was similar across treatments.
Germination percentage was higher and faster for Echinocactus platyacanthus, Ferocactus pilosus, Lepidium virginicum, and Nassella tenuissima, with some of the hydration/dehydration treatments. Atriplex canescens, Cucurbita foetidissima, and Sartwellia mexicana had similar germination across treatments. The differences in germination abilities of the studied species with hydration/dehydration cycles, suggests E. platyacanthus, F. pilosus, L. virginicum, and N. tenuissima are better adapted for germination under limiting soil moisture conditions.
Hydration-dehydration-rehydration cycles are likely to be important in natural seed populations of some species by reducing the time lag between the occurrence of favorable germination conditions and actual germination (Dubrovsky, 1996). Incremental repair of damaged DNA during hydration phases, and the stability of repaired DNA during rehydration phases may effectively shorten the minimal time for germination when seeds eventually receive sufficient moisture to complete germination (Adams, 1999).
Results found here imply that four of the nine studied species respond to hydration/dehydration memory by having more seeds germinated and in at a higher rate. Thus, small precipitation events may have a cumulative effect on seeds, resulting in final germination after only one small rainfall event.











 nueva página del texto (beta)
nueva página del texto (beta)


