Mistletoes are angiosperm plants from the Santalales order, characterized for adhering to a host plant’s stems (Mathiasen et al., 2008). Most mistletoes share an obligate hemi-parasitism lifestyle with specialized structures called haustoria. They take water and minerals from other plants but despite being partially autotrophic, they are still dependent of their hosts (Watson, 2001; Leimu, 2010). This parasite type operates in the community as a plant, but also in a higher trophic level using resources of other plants and therefore, can be compared with herbivores (Pennings and Callaway, 2002). Establishment and survival of mistletoe seedlings is highly dependent on host suitability (Norton and Ladley, 1998). The majority of the parasitic plants have a broad host range, but they can show a specialist behavior developing better in some hosts species in the community (Norton et al. 1995; Press and Phoenix, 2005). Host selection can be influenced by local conditions, such as the type and number of host species available for colonization (Hoffmann et al., 1986; Medel et al., 2004), host structure and characteristics like height (Sales-Teodoro et al., 2009), twig size (Sargent, 1995), and bark type (Arruda et al., 2006). The dynamics of the community can also influence the mistletoe-host relationship, by having more mistletoe occurrence on susceptible host individuals and increasing mistletoe densities, which in turn, increase the fruit production attracting more dispersers reinforcing the contagious distribution (Overton, 1994; Dean et al., 1994; Downey et al., 1997; Aukema and Martínez del Río, 2002b).
Mistletoes also show close interactions with their pollinators and seed vectors, associations that could be considered as a true mutualism. Therefore, mistletoes can act as hemiparasites (for plant hosts) and mutualists (for dispersers and pollinators) simultaneously in natural communities (Watson, 2001). Species of the genus Psittacanthus Mart. are typically dispersed by birds feeding on its fruits that then fly to another tree, voiding the seed on a suitable branch initiating infection. Incubation and production of the first flowers require several years and once established, however, the infection is perennial, and the mistletoe produces large haustoria with many long branches (Vázquez-Collazo and Geils, 2002).
Vázquez-Collazo and Geils (2002) reviewed several reports of Psittacanthus calyculatus (DC.) G. Don having a wide distribution in different climate, vegetation types, and multiple hosts. Huerta-Martínez and Cházaro-Basañez (1997) documented the presence of P. calyculatus in a tropical deciduous forest (TDF) with a distribution from 1,300 to 2,350 m altitude, and found 20 host species. Besides temperate forests and subtropical scrublands, P. calyculatus can occur on hillsides, flat terrain, road edges, irrigation channels, and disturbed areas (Bello-González, 1984), suggesting that the presence mistletoe can be directly or indirectly influenced by human activities (Donohue, 1995), and that high densities of the parasite could indicate a disturbance in the ecosystem.
Although mistletoes can cause adverse effects to hosts by taking essential resources for growth and reproduction or competing for pollinators (Medel, 2000; Sinha and Bawa, 2002; Ollerton et al., 2007), they may also act as keystone resources in forest ecosystems, providing food or nesting sites to a wide range of vertebrates (Watson, 2001). Therefore, we cannot contemplate long-term mistletoe management as pests or threatened plants without considering their ecosystem context (Norton et al., 1997). The objectives of this study were: (1) to determine differences in species composition between a TDF with high density populations of Psittacanthus calyculatus compared to a TDF lacking mistletoe presence, (2) to determine the spatial patterns of mistletoe and its hosts and quantify mistletoe-host association using spatial analysis, and (3) to record avian frugivores in order to establish plausible disperser species in TDF.
Methods
Study site. The study was conducted in a tropical deciduous forest (TDF) located at the urban fringe of Queretaro City, Mexico (20° 30’and 20° 56’ N, 100° 17’ and 100° 36’ W; elevation 1,700 to 2,800 m a.s.l.) (INEGI 2005) from May 2011 to February 2012. Mean annual temperature is 17.6 °C and mean annual precipitation range from 549.3 mm (Baltazar-Ramirez et al., 2004). The climate is typically Aw type of Köeppen classification system, with a six-month rainy season from May to October (Zamudio et al., 1992). The families better represented in the arboreal vegetation are Leguminosae and Burseraceae (Zamudio et al., 1992). The first study site (1) was located Northeast of Queretaro City at the border of Fray Junipero Serra beltway (20° 39’ 40’’ N, 100° 21’ 40’’ W), exhibited disturbance resulting from highway construction and wood extraction and a clear high density of Psittacanthus calyculatus (personal observation). The second site (2) (20° 40’ 38.83’’ N, 100° 25’ 16.75’’ W) was located at the same altitude, west from the first site and exhibited a much lower disturbance than the first site. The third site (3) “El Tángano” (20° 32’ 37.41’’ N, 100° 20’ 22.32’’ W) was found southeast from the city and the area has been considered a natural protected area with the lowest level of disturbance and lacking mistletoe populations.
Diversity analysis. Tree height and canopy cover, as well as individuals of Psittacanthus calyculatus and cover values were taken from the spatial analysis plots (a single 50 × 50 m plot divided in 5 × 5 m subplots) then we computed this data to obtain Shannon and Simpson diversity indices.
Plant community composition. Individuals, species and canopy cover were recorded for all trees measuring > 10 cm DBH inside the 20 × 50 m plot (D). A 100 m2 subplot (5 × 20 m) (C) is centered inside the main plot, all trees > 5 cm DBH were measured and identified. Two 10 m2 and 1 m2 plots were arranged systematically inside the perimeter of main plot. For the two 10 m2 subplots (B) all small trees > 1 cm DBH were measured as well. In 1 m2 subplots only herbaceous plants were identified and coverage measured in percentage. This Modified-Whittaker nested vegetation plot design is described graphically in Stohlgren et al. (1995). Using Biomon software designed by the Smithsonian Institute for the modified Whittaker plots, dominance and density were computed for each tree species. Relative dominance, density and frequency were calculated and added in order to obtain importance value index (Stoholgren et al. 1997).
Spatial Analysis. For the spatial analysis only two contrasting sites were considered using the spatial analysis plots for site “1” with high densities of mistletoe population and site “3”, which was an unaffected site in a protected area. One plot of 50 × 50 m divided in 5 × 5 m subplots were used per site. Within each subplot, the presence and absence of tree species and mistletoe were recorded.
Pattern analyses were conducted using the method of spatial analysis by distance indices (SADIE), developed by Perry (1998). The basis of SADIE is to quantify the spatial pattern in a mapped population by measuring the total effort (in terms of distance moved “D”) that the individuals in the observed sample must experience to move to extreme arrangements, in which the individuals are either spaced as uniformly or are as aggregated as possible. The degree of non-randomness within a set of data is quantified by comparing the observed spatial pattern with rearrangements in which the sampled counts are randomly redistributed among the units (Perry, 1998). Division of “D” by the average value obtained from permutations where the values of the variable under study are randomly arranged among the sampling locations gives an index of aggregation, Ia, which quantifies the spatial pattern. An aggregated sample has an Ia > 1; a random sample has an Ia = 1; and a regularly distributed sample has an Ia <1. The higher the Ia, the more spatially aggregated the community under study. SADIE generates an aggregation index Ia, which is the quotient between observed D and expected D, which is the mean of the data permutations.
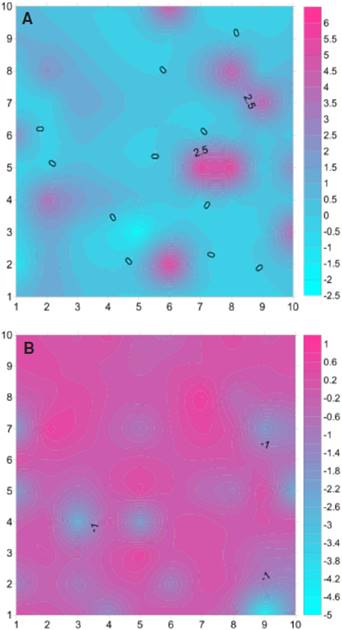
Figure Spatial associations maps of P. calyculatus with (A) Acacia schaffneri Xi=0.6325 and (B) Bursera fagaroides Xi=-0.3208 in a 2500 m2 plot of a Seasonally Dry Tropical Forests in central México. Association values are represented from de lowest (blue) to the highest (magenta).
SADIE methods also provide an association index, high values of local association are indicated by the coincidence of a patch cluster for one set with a patch cluster for the other, or by the coincidence of two gaps whereas dissociation is indicated by a patch coinciding with a gap. The randomization method allows the construction of a test and critical values. Because the method allows for the spatial pattern of each of the two component populations by conditioning on the cluster indices, there should be no relationship between spatial pattern and spatial association (Perry and Dixon, 2002). The dimensionless indices for clustering, Vi and Vj quantify the degree to which the count for each sample unit contributes towards the overall degree of clustering, as part of a patch or as a gap. Their overall mean values (υi, υj) may be used to formally test for clustering within one set, and compare informally different sets of data (Perry et al., 1999). SADIE analyses were conducted with SADIEShell v 1.22 (©Kelvin F. Conrad and IACR-Rothamstead). Indices Ia, υi, υj and association index with their probabilities were calculated for tree species and Psittacanthus calyculatus. Local aggregation indices (υi and υj) and the association index or spatial covariation among pair of variables (X) defined by Perry (1998) were used to depict association maps with the software SURFER v. 8.02-Oct 2001 (Surface Mapping System© 1993-2002, Golden Software, Inc.).
In order to obtain cover values we measured maximum canopy diameter and a second diameter in perpendicular position, then we calculated canopy area using the formula for the area of an ellipse, i.e.,
Where r1 and r2 are the two radii.
Bird feeding survey. In order to find plausible dispersers of Psittacanthus calyculatus seeds, observation points were established at site 1 where high incidences of mistletoe were observed. Observations were made from 8,000 h to 1,200 h during January and February 2012 (a total of 44 h), when ripe fruits were available. Bird species and fruit interaction behavior were recorded, as well as the tree species where birds perched.
Results
Diversity analysis. We recorded seven tree species in site “1”, 12 in site “2”, and 10 in site “3”. At site “1” only three species were found with mistletoe: Acacia schaffneri, A. farnesiana, and Prosopis laevigata. The proportion of infected host trees was 71.42 % of A. schaffneri (N = 7), 62 % of A. farnesiana (N = 10), and 16 % of P. laevigata trees (N = 30). A total of 165 individuals of Psittacanthus calyculatus were recorded. Mistletoes were distributed at 44.84 % (N = 74) in A. schaffneri hosts, 41.81 % (N = 69) in A. farnesiana, and 13.33 % (N = 22) of mistletoe plants in P. laevigata. The conserved TDF (site 2 and 3) had greater values in richness and diversity indices for the vegetation (Shannon = 1.68, Simpson = 0.757 and Shannon = 1.294, Simpson=0.553 respectively) compared to a disturbed TDF (site 1; Shannon = 0.987; Simpson = 0.476).
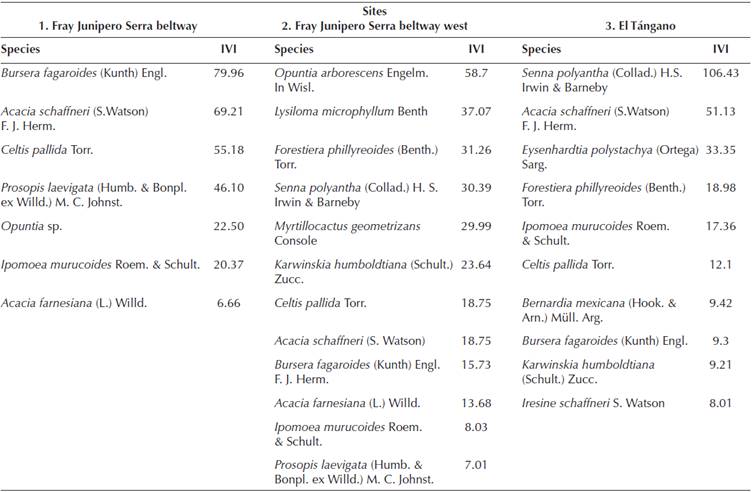
Plant community composition. The community composition of the study sites differs resulting from the impacts of human activities. Bursera fagaroides and Acacia schaffneri importance values switch drastically between sites “1” to site “2” (Table 1). From being the two species with the higher values, they change to be within the last four species in the preserved TDF. Acacia schaffneri keeps the second place in importance in site “3”, but considering the importace value relation, the value is the half of Senna polyantha.
Table 1 Importance value indices (IVI) of tree species in three sites of Seasonally Dry Tropical Forests in central México.
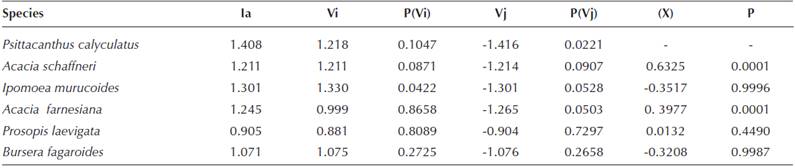
Spatial Analysis. The aggregation index (Table 2) indicates clearly that Psittacanthus calyculatus has an aggregated pattern (Ia = 1.408) as well the main host trees: Acacia schaffneri (Ia = 1.211) and A. farnesiana (Ia = 1.245). Strong spatial associations of the mistletoe with both A. schaffneri (X = 0.6325, P = 0.0001) and A. farnesiana (X = 0.3977, P = 0.0001) were found, in contrast with Ipomoea murucoides (X = -0.3517, P = 0.9996) and Bursera fagaroides (X = -0.3208, P = 0.9987), which showed an evident dissociation. These data indicate that P. calyculatus has a narrow host range within the TDF studied.
Table 2 Aggregation and association indices of Psittacanthus calyculatus and main tree species in a 2,500 m2 plot in a Tropical deciduous forests in central Mexico. Indices indicate Aggregated distributions when Ia > 1, random distributions if Ia = 1 and regular distributions if Ia < 1; Vi = patches, Vj = gaps. X = association index. P = two tail test α = 0.05. Associated X = > 0; Dissociated X = < 0; random arrangement X = 1.
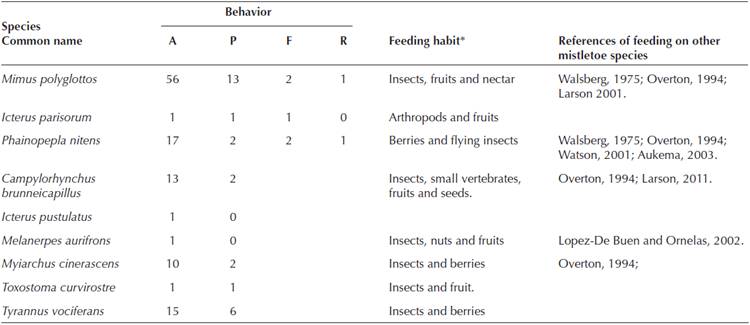
Bird feeding survey. A total of 195 observations were made and 28 bird species were registered. Only ten species interacted with Psittacanthus calyculatus whether perching or eating the mistletoe berries. Three species were observed consuming the fruits: Mimus polyglottos, Phainopepla nittens, and Icterus parisorum, the first two regurgitated the seed in one occasion. Mimus polyglottos had the higher perching frequency. Disperser candidates were considered by feeding habits, size and for being reported as mistletoe fruit consumers (Table 3).
Table 3 Psittacanthus calyculatus’ disperser candidate’s abundance and direct interactions with it, such as perching, feeding and regurgitation in a Tropical deciduous forests in central Mexico. A = abundance, P = perching, F = feeding, R= regurgitation. *Ehrlich et al. (1988).
Discussion
Differences in plant communities. The results show a narrow host range for Psittacanthus calyculatus in the tropical deciduous forest (TDF) of the present study, with three host species in the Leguminosae family: Acacia schaffneri, A. farnesiana, and Prosopis laevigata. These results contrast to hosts species reported by Huerta-Martínez and Cházaro-Basañez (1997) who found 20 host species in a TDF and a Quercus forest in Sierra de Tapalpa, Jalisco, where the most frequent hosts belonged to three families, which included Leguminosae, and the host species present in this study: Acacia spp. and P. laevigata.
Most parasite populations depend on the availability of the host (Donohue, 1995). The changes in importance values of the hosts between the infested and non-infested TDF, suggests a link in the presence of Psittacanthus calyculatus and the composition of tree community. Roxburgh and Nicolson (2005) found that the infection prevalence of the mistletoe Plicosepalus kalachariensis which had high host specificity, was related with host abundance. The impact of parasitic plants on a community may be widespread, especially if the most parasitized plant species are dominant (Press and Phoenix, 2005). This suggests that communities where mistletoe hosts have high importance values can be more vulnerable to colonization and mistletoe spreading.
Spatial aggregation and association of Psittacanthus calyculatus and hosts. Aggregation in mistletoes on their hosts is very common and this clumped distribution have been detected in other mistletoe species, such as Phoradendron (Larson, 1996; Aukema and Martínez del Río, 2002a; Aukema, 2004), Tristerix aphyllus (Medel et al., 2004), Tristerix corymbosus (García et al., 2009), and Tristerix verticillatus (Lemaitre et al., 2011). As expected, P. calyculatus has an aggregated pattern among it hosts in the present studies. The aggregated spatial arrangement can be found in other Psittacanthus species like P. robustus in South East Brazil, (Ferreira-Monteiro et al., 1998; Sales-Teodoro et al., 2010), P. biternatus, P. plagiophyllus and P. eucalyptifolius in North Brazil (Fadini and Lima, 2012).
Bird vector can have an important influence in favoring an aggregated distribution of mistletoe (Aukema and Martínez del Río, 2002b). According to Sales-Teodoro et al. (2010), host distribution and behavioral patterns of seed dispersers are directly responsible of mistletoe distribution patterns. Vectors are attracted by the presence of mistletoe increasing the parasite aggregation, then a group of mistletoes with different origins could share the same host, subsequently increasing the visibility to other birds (Larson, 1996; Medel et al., 2004), reinforcing a spatial mistletoe-disperser interaction cycle, whereby infected trees receive an escalating number of seeds (Overton, 1994).
Both Acacia species showed an aggregated arrangement. If host trees are considered as living patches and they are only substrate where mistletoe population can inhabit (Overton, 1994), a host-clumped distribution will facilitate dispersion providing a continuous substrate and subsequently enhancing mistletoe spatial aggregation. Also, tree spatial patterns can influence frugivore foraging decisions, in Carlo and Morales (2008) model when fruiting plants become aggregated, unevenness in fruit-removal rates increases and seed dispersal distance decreases, due to birds moving shorter distance in high density neighborhoods. This can be a balancing factor between aggregated distribution and dispersion efficiency.
Acacia schaffneri was the host with higher index of association with Psittacanthus calyculatus. Dean (1994) registered 24 mistletoes species as parasites of the genus Acacia considering it the most important host in South Africa. Species of the genus are usually described as the main host (Bowie and Ward, 1994; Donohue, 1995; Roxburgh and Nicolson, 2005) or as frequent hosts (Barlow and Wiens, 1977; López de Buen and Ornelas, 2002; Dzerefos et al., 2003). On the other hand, Acacia species are an integral component of plant communities in semiarid climates (Zamudio et al., 1992) with drought tolerance (Clemens and Jones, 1978) that permits individuals to resist the six to seven months of the dry season of the TDF. Since mistletoes of Loranthaceae family have a higher transpiration rates than their hosts (Ulman et al., 1985), Acacia and Prosopis trees can be convenient hosts because are phreatophytic species capable of providing water throughout the year due to an extensive root system that extends both deep and wide (Ludwig et al., 2003).
Bird feeding survey. Psittacanthus seeds are dispersed by several species of birds, especially fly-catchers, thrushes, and tanagers (Genini et al., 2012). Psittacanthus calyculatus fruit production starts primarily during the dry season (Vázquez-Collazo and Geils, 2002) and begins in January and extends to May when most of TDF tree species remain leafless (Zamudio et al., 1992) and insect abundance is low. This is very convenient for insectivore-frugivorous birds. López de Buen and Ornelas (2001) studied the synchrony between abundance of P. schiedeanus ripe fruits and abundance in fruit eating birds, where the fruit phenology was very important for birds with generalist feeding habits. This can also benefit neotropical migrants such as Icterus spp. López de Buen and Ornelas (2002) observed Icterus galbula consuming P. schiedianus fruits. Phainopepla nitens a Silky-flycatcher from Central Mexico is well known as the main disperser of the desert mistletoe Phoradendron californicum (Walsberg, 1975; Larson, 1996; Aukema and Martínez del Río, 2002a). The Phainopepla’s intestinal tract is highly adapted for processing mistletoe seeds (Walsberg, 1975). However, there are no studies documenting P. nitens consuming Psittacanthus calyculatus fruits. It seems that P. nitens can benefit from P. calyculatus spatial aggregation, because this species defines territories in function of resource agglomeration and defends mistletoe clumps from other foraging species (Walsberg, 1975).
Mimus polyglottos the Northern mockingbird is considered part of the generalist assemblage of birds feeding on Phoradendron californicum, which can provide appropriate dispersal for some seeds (Larson, 1996). The high territorialism in M. polyglottos (Farnsworth, 2011) and an apparent fearless behavior towards man, could explain the numerous sights of perching in P. calyculatus. Like P. nitens, Northern mockingbirds haven´t been considered as a Psittacanthus disperser.
The interactions of Psittacanthus calyculatus with hosts plants and avian vectors data shows that host availability and spatial arrangement are important factors shaping mistletoe distribution; also low tree diversity is related to high mistletoe densities and a strong dominance by hosts can lead to a greater rate of infection (Press and Phoenix, 2005). Distribution of host species can partially determine mistletoe distribution patterns (Overton, 1994) and host aggregation can further aggregate mistletoe on a greater spatial scale. Although P. calyculatus has been reported to have multiple hosts throughout different vegetation types, the species was highly associated with only two trees: Acacia schaffneri and A. farnesiana. Of the bird species observed, M. polyglottos and P. nittens frequent P. calyculatus using it as a food resource, behavioral observations and background with other mistletoe species, indicate that both can be efficient seed dispersers. Further analyses are needed to establish dispersion roles on the P. calyculatus vector ensemble in TDF.











 nueva página del texto (beta)
nueva página del texto (beta)


