Coniferous forests represent 17 % of the Mexican territory. About 90 % of this area comprises Pinus or Pinus-Quercus species association (Rzedowski, 2006). There are approximately 50 pine species described in Mexico, which represents more than 50 % of all pine species around the world, many of these with distribution restricted to the country (Nieto de Pascual-Pola, 2009; GD, 2013). Moreover, pines can be distributed over a wide variety of climatic, topographic, and geologic conditions, ranging from cold to warm weathers, and from high mountains to the sea level (Rzedowski, 2006). Hartweg’s pine (Pinus hartwegii Lindl.) forests are of great ecological importance because they are the arboreal vegetation most tolerant to low temperatures and high altitudes, reaching the treeline limit at 4,000-4,200 m a.s.l. (Musalem and Solís, 2000; Rzedowski and Rzedowski, 2005; Rzedowski, 2006). Therefore, Hartweg’s pines are distributed in almost all high mountain areas and peaks of Mexico, and they tend to form large monospecific stands (Musalem and Solís, 2000).
The main biotic agents that impact Pinus hartwegii growth are intraspecific and interspecific competition (Zepeda and Villarreal, 1987; Santillán, 1991; Geils and Hawksworth, 2002; Kunstler et al., 2012), seed and cone predation by rodents (Musalem and Solís, 2002), and forest diseases (Musalem and Solís, 2002; Cibrián et al., 2007). Among the principal forest diseases on P. hartwegii are enlisted insects, such as the Scotylid bark beetles, Torymidae wasps and Pyralidae moths, fungi (such as Ganoderma spp., Poliporus spp. and Amillaria spp.), and dwarf mistletoes (Musalem and Solís, 2002; Cibrián et al., 2007).
Dwarf mistletoes (Arceuthobium spp., Viscaceae) are hemiparasitic plants and one of the most relevant disease causing agents in pines of the North American temperate forests (Shaw et al., 2008; Mathiasen et al., 2008). These parasitic plants are of ecological importance because they play a key role by providing resources, such as shelter and food, to different animal species enhancing community diversity (Watson and Herring, 2012; Chávez-Salcedo, 2013). Moreover, they are of economic importance because their infection causes a reduction in growth and fitness in the host trees leading to a major forest product loss (Hawksworth and Wiens, 1996; Heide-Jorgensen, 2008). In Mexico, there is an annual loss of 2.0 × 106 m3 of roundwood (Vázquez, 1993), whereas the loss in Western USA and Canada has been estimated as 11.3 × 106 m3 and 3.8 × 106 m3, respectively (Heide-Jorgensen, 2008).
The stand structure is strongly associated with dwarf mistletoe dynamics, where it has been shown that the crown volume and size of the dominant cohort decreases with infection intensity (Shaw et al., 2005; Agne et al., 2014). These hemiparasitic plants form a complex root-like structure, called haustorium, which is the organ of contact with the hosts’ xylem and phloem (Hawksworth et al., 2002). Through this connection, mistletoes take mineral nutrients, water and, although they have a photosynthetic capacity, most of their organic nutrients (Press, 1995). The latter have important effects on host performance, causing decreased growth, branch and stem deformations, reduced water use efficiency, decreased photosynthetic capacity, decreased fecundity and, with severe infection, death of the host (Geils and Hawksworth, 2002; Meinzer et al., 2004; Mathiasen et al., 2008). The impact can go from negligible to severe, depending on the level of the infection and the development status and vigor of the host (Musselman and Press, 1995). Some studies report that the effect on the hosts’ growth is unnoticeable until the infection is severe (Hawksworth and Wiens, 1996; Geils and Hawksworth, 2002; Shaw et al., 2008).
A decrease in the growth of the host has been recorded for different morphometric variables: diameter at breast height (dbh), tree height, volume, and basal area (Hawksworth and Wiens, 1996; Geils and Hawksworth, 2002; Shaw et al., 2008). Andrade and Cibrián (1980) report a reduction of 19 and 29 % in dbh and height, respectively, in Pinus hartwegii individuals parasitized by Arceuthobium spp. compared with healthy individuals. In other conifers, the reduction in parasitized individuals’ dbh due to different dwarf mistletoe species ranges from 2 to 56 %, where growth decrease is non-linearly related to infection class; that is, slight reduction is produced with light infection, but there is a threshold of severe infection after which reduction increases rapidly (Hawksworth and Wiens, 1996; Geils and Hawksworth, 2002). Similar results have been reported for the hosts’ volume and basal area (Hawksworth and Wiens, 1996; Madrigal et al., 2007; Shaw et al., 2008).
Hartweg’s pines are not only of ecological importance but also of an economic one (Eguiluz, 1978; Hernández, 1985; Musalem and Solís, 2002). Forest diseases, such as dwarf mistletoes, lessen their health for which it is important to investigate their effect in order to include this last aspect for management and reforestation plans. Although the effect of dwarf mistletoes on host performance has been widely studied, the joint effect of different species parasitizing the same host tree has not been investigated. In Zoquiapan (Central Mexico), two species of dwarf mistletoe, Arceuthobium globosum Hawksw. & Wiens and A. vaginatum J.Presl., coexist parasitizing Pinus hartwegii on the same areas and even on the same individual host, showing an aggregated pattern within the host (Queijeiro-Bolaños et al., 2014). Both mistletoe species have a complex interaction within each other, as they compete for host resources; however, under particular circumstances, they also facilitate each other’s colonization (Queijeiro-Bolaños et al., unpub. data). The latter makes us think that the effect of these species parasitism may be different when just one or two species are infecting the host. The objectives of this study were: (1) to evaluate the isolated and the combined effect of two dwarf mistletoe species infestation (Arceuthobium globosum and Arceuthobium vaginatum) on the growth of Pinus hartwegii, (2) to assess whether the allometric relationship between height and diameter-at-breast-height of the pines is modified by the infestation, and (3) to recognize what host size is more susceptible to the infection.
Material and methods
Study area. The study was conducted in the Zoquiapan portion of the Iztaccíhuatl Popocatépetl National Park, State of México. It is located on the East Central part of the Trans-Mexican Volcanic Belt and comprises the main part of the Sierra Nevada (SEMARNAT, 2013). The study site was located at one side of the Southern slope of the Papayo Hill (19° 18’ 08.4’’ N, 98° 42’ 10.7’’ W, 3,420 m a.s.l.). The climate is temperate sub-humid with summer rains, mean annual temperature is 9.8 °C (range: 1.3-18.3 °C), and annual rainfall is 941 mm, with duration of the rainy season being June to September (SMN, 2013). Vegetation is classified as high mountain temperate forest, dominated by large extensions of Pinus hartwegii stands (Arriaga et al., 2002); other arboreal species present are Abies religiosa, Alnus jorullensis, Cupressus lusitanica, Pinus ayacahuite, P. leiophylla, P. montezumae, P. pseudostrobus, Quercus crassipes, Q. laurina, and Q. rugosa. Tussocky grasses, such as Muhlenbergia macroura, M. quadridentata, Calamagrostis tolucensis, and Festuca tolucensis, are the most frequent and conspicuous plant species on the understory (Obieta and Sarukhán, 1981; SEMARNAT, 2013).
Study species. Hartwegs’ pine or Mexican mountain pine, Pinus hartwegii, according to Musalem and Solís (2000) and Rzedowski and Rzedowski (2005), is typical of Mexican mountain ranges on altitudes from 2,600 to 4,200 m, being most frequent on the Sierra Nevada region. It is a tree of 15-30 m in height and up to 75 cm dbh. The bark is thick, rough and scaly, and dark brown to gray in color. Branchlets are stiff, curving upwards with persistent leaf bases. Needles in fascicles are presented in groups of three, although sometimes five, of 6-18 cm length. Cones are grouped in two-six, and are obliquely ovoid, 7-14 cm, and reddish to almost black in color. The seeds are brown, 5-8 mm long, often having black spots, and wings of 12 × 20 mm. Generally, it can be distributed in monotypic stands, but in its lower altitudinal range it can be found coexisting with other trees, such as Abies religiosa and Alnus jorullensis.
In Zoquiapan, Pinus hartwegii is parasitized by Arceuthobium globosum subsp. grandicaule Hawks. & Wiens and A. vaginatum subsp. vaginatum (Mexican dwarf mistletoe), which is the zone where the three species converge (Hawksworth and Wiens, 1996; GD, 2013). The two mistletoe species have similar features: dioecious plants, with sexual dimorphism; anemophilous pollination and ballistochoric dispersal; and seven host species in common (Pinus hartwegii, P. durangensis, P. lawsonii, P. montezumae, P. patula, P. pseudostrobus, and P. rudis). Arceuthobium globosum is 18-70 cm tall with yellow-greenish shoots; it is distributed from Central Mexico to Guatemala. Arceuthobium vaginatum is 20-55 cm tall with brown-blackish shoots; tt is distributed from Northern to Southern Mexico (Hawksworth and Wiens, 1996; Cibrián et al., 2007).
Sampling and data analysis. In a 1.1 ha monotypic stand, we selected pine trees > 2 m and 2.5 cm of diameter at breast height, because smaller trees are rarely infected by dwarf mistletoe (< 2 %; Hernández-Benítez et al., 2005). The stand has an average of 55.9 % of trees infected by Arceuthobium globosum and 22.9 % by A. vaginatum (Queijeiro-Bolaños et al., 2014). A total of 161 pines of different size and infection condition (i.e., pines infected by A. globosum, A. vaginatum, both species, or neither) were selected. From November 2008 to November 2011, we measured diameter at breast height (dbh), tree height, and average crown spread for each individual, every six months. The average crown spread was obtained by measuring the longest extent of the crown and the longest cross-extent, and then averaging the two measures (Blozan, 2004). The height was estimated by calculating the angle (α) with a clinometer from the observer to the tip of the tree, and then the distance (d) from the observer to the tree. The height was estimated by using the formula h = d × tan(α).
We also measured the infection intensity with a modified dwarf mistletoe rating system (MDMR), based on Hawksworth’s method (Hawksworth, 1977). In our system, the tree was vertically divided into thirds, and each third was rated into one of the following classes: 0, no infection; 1, sprout or hypertrophy; 2, less than 20 % of branches infected; 3, 21 to 30 %; 4, 31-50 %; 5, 51-70 %; and 6, more than 71 %. The three thirds were summed for each individual, so the scale went from 0 to 18.
To evaluate the isolated and the joint effect of the dwarf mistletoe infection on Pinus hartwegii growth rate, we first tested with repeated measures ANOVA (Zar, 2010) whether there was a significant change in dbh, height, and crown spread over the three years. Because only dbh showed a significant change (Appendix 1), we computed the pines’ annual relative growth rate (RGR) using dbh. We first analyzed separately, if there were significant differences between the infecting species (i.e., both species, Arceuthobium globosum, A. vaginatum, or none) on dbh RGR and initial dbh by one way ANOVA and on MDMR by Kruskal-Wallis test. We then performed a linear model including, as main effects, the infection severity (MDMR) and mistletoe species (none, A. globosum alone, A. vaginatum alone, or both coexisting mistletoes), and pines’ initial dbh as a covariate, as it was found to be related to RGR (See Results section). We used the records of MDMR and infecting species at the beginning of the study, because RGR for the three-year sampling must be a response of the previous conditions of parasitism, and three years is a short time to notice a significant change on MDMR or species present (linear analysis of RGR with final MDMR and species, and also with the average MDMR and species can be found in the Appendix 1).
To determine whether the relationship between height and dbh was modified by the infection, we fitted the Chapman-Richards model (Peng et al., 2001) with non-linear least squares method (nls) for the following four types of infection: pines parasitized by Arceuthobium globosum, A. vaginatum, and both, or neither. Chapman-Richards is a non-linear function that describes the changes in the relative dimensions of different parts of the pines, in this case height and dbh. The simplest form of the equation is as follows:
where y refers to the tree height, x is dbh, and α, β, and γ are the parameters of the asymptote, the rate and the form (Richards, 1959; Chapman, 1961). Peng et al. (2001) added dbh (1.3 m) to the function, to ensure that the total height equals 1.3 m when dbh is 0:
The parameters of the four fitted curves were tested against a null model where the data sets of each condition (none, both species, Arceuthobium globosum, or A. vaginatum) shared global parameters (i.e., a single curve for all the data), and tested with an extra sum of squares F test (Motulsky and Christopoulos, 2003) with Prism 6 (GraphPad Software, 2014).
In order to know which host size is more susceptible to infection, either by one species or both, we prepared 7 × 4 contingency tables, with seven size categories based on crown spread (height and dbh were non-significant; hence it is not shown in the results) and four infection conditions (i.e., infecting species: no infection, Arceuthobium globosum, A. vaginatum, or both species). Moreover, we prepared a 7 × 4 × 2 contingency table with the same seven size classes, four levels of infection [based on MDMR values: none, light (rates 1, 2, and 3), moderate (4, 5, and 6), and severe (7, 8, 9, and 10, because rates 11 to 18 were not found)], and two dwarf mistletoe species. For each contingency table, we performed a generalized linear model (glm) with Poisson error distribution and log link function (Crawley, 2007).
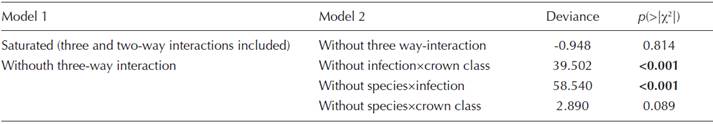
For tridimensional contingency tables, we first fitted the saturated model, where the main effects and the two and three-way interactions were present. Later, we compared it with models lacking the three and two-way interactions with a log-likelihood test (Crawley, 2007). Thereafter, we verified trough Pearson residuals whose categories were significant. All analyses were performed using the stats package, except for Pearson residual analysis, which was performed using the vcd package (Meyer et al., 2006, 2012; Zeileis et al., 2007) both of the R software (R Development Core Team, 2012).
Results
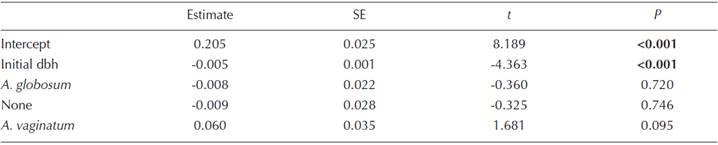
The relative growth rate (RGR) of Pinus hartwegii was strongly correlated with the pines initial dbh, where there was a clear tendency of a decrease in RGR as the trees become larger (r 2 = 0.2, P < 0.001; Figure 1). There were significant differences according to the parasitizing species (both, Arceuthobium globosum, A. vaginatum, or none), on dbh RGR (F (3,123) = 2.3, P < 0.0001; Figure 2A), MDRM (χ2 = 32.46, d.f. = 2, P < 0.0001; Figure 2B), and initial dbh (F (3,126) = 10.068, P < 0.0001; Figure 2C). Trees infected by A. vaginatum showed larger RGR, followed by none-infected trees and trees with A. globosum, whereas trees with both mistletoes did not differ with those infected with A. globosum or uninfected trees (Figure 2A). On the other hand, trees infected by both species had a larger MDMR than each dwarf mistletoe alone (Figure 2B). In addition, the initial dbh of trees with both species was significantly larger than that of trees with A. vaginatum alone and the non-infested ones, but did not differ from trees with A. globosum alone (Figure 2C). Because we knew that the results of RGR could be misled by the influence of the initial size of the tree, we performed a linear model that included the effect of dbh as a covariate; only the initial dbh turned out to be significant (Table 1). That is, neither the slope nor the intercepts of RGR against initial dbh vary with the infecting species (Table 2, Figure 3). We observed that the RGR of uninfected trees or those infected by both or one species follow the same pattern of decrease with the size described before (Figure 1), suggesting no effect of the parasites.
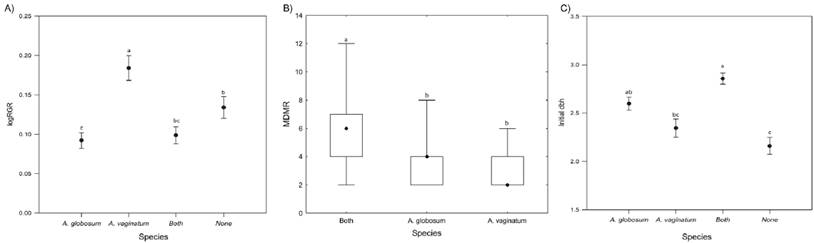
Figure 2 Separate effect of parasitizing species, Arceuthobium globosum, A. vaginatum, both, or none, on the following aspects: A) RGR (F 3, 123 = 9.718, P < 0.0001); B) mean MDRM (F 2, 87 = 26.305, P < 0.0001), C) initial size (F 3, 126 = 13.649, P < 0.0001). Letters above the boxes indicate significant differences according to Tukey’s HSD test.
Table 1 Analysis of covariance of the effect of parasitizing species, MDMR, and initial dbh on pines RGR.
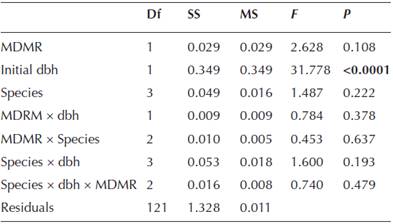
Table 2 Summary of the parameters of each level of the factor Species on RGR in the model RGR = β0 + β1dbh. No significant differences were found.


Figure 3 RGR with different initial dbh and different parasitizing species. Arceuthobium globosum: ▲ symbols and dashed line; A. vaginatum: × and dot-dash line; both species: ● and solid line; none: + and dotted line.
The allometric relation between tree height and dbh was different for each type of infection (none, both species, Arceuthobium globosum, or A. vaginatum; F 9,1291 = 2.072, P = 0.029; Figure 4). After fitting the Chapman-Richards model (eq. 2) and plotting the predicted values and their confidence intervals (Figure 4), some patterns could be distinguished. For dbh < 30 cm, there are no clear differences between the infection conditions because the confidence intervals of the four curves overlap; however, on trees > 30 cm dbh, the curves start separating, although their intervals are still overlapped. On trees between 30 and 40 cm dbh, the curve of “both species” begins to look lower than the rest. Between 40 and 60 cm, it is noticeable that the curves of “none” and “A. vaginatum” completely overlap, showing no differences in height at those dbh sizes; however, the height of “both species” is considerably lower than the latter two, barely overlapping with those two intervals. Arceuthobium globosum showed a great variation; although the curve seems to be below the “none” and “A. vaginatum” and above both, the confidence interval is too wide to conclude any difference from the other curves.

Figure 4 Allometric relation between Pinus hartwegii dbh and height. Dashed lines represent confidence intervals for each curve; Arceuthobium globosum: blue; A. vaginatum: orange; both species: red; none: green.
We tested which crown spread classes were more frequently infected; dbh and height classes were non-significant, hence the results are not reported. In addition, crown spread is a better descriptor for mistletoe presence because it represents the seed “landing” area. There is a significant association between crown spread and infection type (χ2 = 45.41, d.f. = 18, P = 0.0003), but only A. globosum showed a significant difference between the observed frequencies and that expected by chance in any crown spread size classes (Figure 5), where trees < 2 m are more frequently infected than those expected by this species and trees of 2-3 m and 6.4 and 7.5 had a lower frequency. On the other hand, it is worth to mention that the MDMR increased its magnitude with crown spread size (i.e., the wider the crown, the more severe the infection would be; Figure 5).

Figure 5 Number of pines on different crown classes (χ2 = 45.421, g.l. = 18, P < 0.001), parasitized by Arceuthobium globosum, A. vaginatum, both, or none (left axis), and mean MDMR of A. globosum and A. vaginatum in this same classes (right axis). (+) symbol indicates a frequency higher than expected, whereas (-) indicates a frequency lower than expected.
The results of the three dimensional contingency table showed that the three-way interaction between crown spread classes, infection levels (none, light, moderate, and severe), and infecting mistletoe species (Arceuthobium globosum and A. vaginatum) was non-significant (deviance = 1.19, d.f. = 3, n.s.); nevertheless, the following two-way interactions were significant: infection × crown spread class, species × infection, and species × dbh class (Table A6 on Appendix 1). According to Pearsons’ standardized residuals, there are some differences between infection level and crown spread (χ2 = 58.355, d.f. = 18, P < 0.0001), where categories moderate or severe of A. globosum have, in general, higher observed frequency than expected by chance in trees greater than 1.96 m (Figure 6A), whereas A. vaginatum light severity is significantly larger only on trees 1.96-3 m, and underrepresented on trees smaller than 1.96 m (Figure 6B). Arceuthobium vaginatum had clearly a lower frequency on Pinus hartwegii, and, although non-significantly, has a greater severity on larger trees.

Figure 6 The percentage of Pinus hartwegii trees on different crown classes and their infection severity (none, light, moderate, or severe) of the following species: A) Arceuthobium globosum and B) A. vaginatum. (+) symbol indicates a frequency higher than expected, whereas (-) indicates a frequency lower than expected.
Discussion
Our results showed that there is no significant effect of the dwarf mistletoe species, either alone or together, on Pinus hartwegii dbh RGR; nevertheless, there is evidence that dbh-height allometric relations are different depending on the infecting condition (either Arceuthobium globosum, A. vaginatum, both, or none). In general, RGR for the four infecting categories follows the same decreasing pattern with dbh. This means that there is no difference between the infected trees (whether these have one or two species) and uninfected ones. The affection of dwarf mistletoes on the hosts’ growth has been a common subject of research [see Geils and Hawksworth (2002) for a summary of works]. However, Geils and Hawksworth (2002) acknowledged that only few generalizations can be made for this parasitic genus, where the effect may depend on a mixture of the following factors: i) host-parasite species combination, where some mistletoe species are more harmful than others and some hosts show a higher resistance to infection (Tinnin, 1981; Hawksworth and Wiens, 1996); ii) severity of infection, because the effect can go from negligible with low intensity to lethal with a large load of parasites (Musselman and Press, 1995; Shaw et al., 2008); iii) host vigor and developmental stage (Tinnin, 1981; Hawksworth and Wiens, 1996); and iv) density-dependent mechanisms and the activity of secondary parasites (Tinnin, 1981).
In the present case, contrary with what has been stated by other authors (Andrade and Cibrián, 1980; Madrigal et al., 2007), it seems that these species, and their combination, are not harmful to pines growth, because there is plenty of evidence that growth is arrested only by age (represented as initial dbh); RGR varies with the ontogenetic condition of the individuals, because there is a decrease in growth rates as the plants increase in biomass (Paine et al., 2012), and this should be considered when modeling plant growth rates. The influence of the initial dbh on some growth measurement has been treated by other authors; for example, Shaw et al. (2008) found that the initial dbh of Pseudotsuga menziesii infected with Arceuthobium tsugense, explained a considerable amount of the variation in the basal area growth of infected trees.
It is worth mentioning that most studies reporting on the growth effect were conducted on even age (or even sized) stands, which provide a control of some variables, such as initial size, but are hardly extrapolated to natural conditions or uneven age stands. We are presenting evidence that patterns drawn for heterogeneous stands can be fairly different from homogeneous stands; moreover, we can argue that hosts growth on a stand with a heterogeneous size structure is not severely affected by dwarf mistletoes, indicating the convenience of maintaining forest heterogeneity.
Although no significant effect was shown on RGR, the allometric curves showed some interesting patterns. In general, trees infected with Arceuthobium globosum and both species were shorter at dbhs > 30 cm, compared with uninfected trees and those infected with A. vaginatum. Although we cannot be certain about the history of the development of these trees and what factors could have affected their growth in the past, we can think of it as a consequence of the presence of mistletoe. Dwarf mistletoes can cause an abnormal biomass accumulation, provoking thicker boles due to hypertrophy or more voluminous crowns due to deformations such as witches’ brooms (Hawksworth and Wiens, 1996). Moreover, the infection of dwarf mistletoes can modify stand structure, where the dominant and co-dominant cohort of pines tend to be shorter than uninfected ones (Agne et al., 2014). In this pine species, infection is fairly common over the stem and could cause trees to still gain bole thickness as a response to infection but not height compared with non-infested trees. As expected, the lesser height was notorious for pines infected with both species and A. globosum; however, trees infected with A. vaginatum showed the same pattern than uninfected trees, demonstrating that the effect of this species could be null when it refers to allometric relationships. It is noteworthy that no differences can be depicted on trees < 30 cm dbh, and this is probably because younger individuals have a greater increment in biomass (Paine et al., 2012), and also because the infection is less severe on these individuals (Figure 5). A non-linear effect has been shown before, where the effect of dwarf mistletoes is not noticeable until the infection is severe (Geils and Hawksworth, 2002; Shaw et al., 2008), and we had a bias toward smaller trees with light or moderate infection, but that is a reflection of the natural stand structure.
With respect to crown spread size susceptibility, some patterns agree with what was expected: most of the uninfected trees were the smallest ones and MDMR increased with tree size (Shaw et al., 2005). Young trees are rarely parasitized because they cannot provide enough resources to the parasite and the consequences for them are mostly lethal (Press, 1995); however, a larger tree represents a better resource because it has a larger surface for seed reception (Arriaga et al., 1988; Hernández-Benítez et al., 2005) and can bear a larger load of parasites declining in health but not in mortality (Musselman and Press, 1995; Hawksworth and Wiens, 1996). From the results, it can be said that trees with a crown spread smaller than 1.96 m were more frequently infected by Arceuthobium globosum (Figure 5), but this species is more severely on larger trees (Figure 6); whereas, the categories A. vaginatum or both species do not seem to be more or less frequent on any size category (Figure 5), but A. vaginatum showed lighter infections on smaller trees and greater infections on larger trees (although this last was not significant, Figure 6). However, infection of trees with A. vaginatum was a lot less frequent than that with A. globosum, suggesting that A. vaginatum might be less harmful. Forest canopy, here represented as crown spread, represents an important resource for mistletoes because it is not only the place for establishment but it also has the suitable conditions for these plants to perform adequately (Reid et al., 1995; Shaw, 2004). Arceuthobium vaginatum is a smaller plant with apparently lesser photosynthetic requirements than A. globosum (Calvin et al., 1984; Hawksworth and Wiens, 1996); thus, A. globosum could be gaining more benefit from larger trees, hence being more severe within these sizes.
The results showed that these parasites are not significantly harmful for host growth, stating the importance of maintaining forest heterogeneity. This scenario where two dwarf mistletoes are coexisting is rare (Hawksworth and Wiens, 1996; Queijeiro-Bolaños et al., 2014); thus, it raises the question about how resources are used and shared among the two species. In addition, although these parasites may not be arresting pine growth in a noticeable way, we cannot state that these have no effect on forest industry activities, such as poor wood quality and lower roundwood production (Andrade and Cibrián, 1980; Madrigal et al., 2007; Logan et al., 2013).
Conclusions
Our results suggest than Arceuthobium globosum and A. vaginatum do not have a joint effect on Pinus hartwegii dbh RGR. These lead us to think that the infection by these two parasites on this area is not detrimental for the host populations. Uneven-aged forests represent a more complex scenario where the severity of infection is commonly greater because there are more spaces to colonize (Shaw et al., 2008). Nevertheless, the latter has been reported for single-species stands with the infection of only one mistletoe species; there are two species present in this area using the same host as a resource, and our results suggest that it may be helpful to maintain the complexity of an uneven-age forest to avoid the dominance of only one mistletoe species, which could be more harmful for the host.











 nueva página del texto (beta)
nueva página del texto (beta)