Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Botanical Sciences
versión On-line ISSN 2007-4476versión impresa ISSN 2007-4298
Bot. sci vol.93 no.4 México dic. 2015
https://doi.org/10.17129/botsci.250
Etnobotánica
Chemical and morphological characterization of Agave angustifolia bagasse fibers
Caracterización química y morfológica de las fibras de bagazo de Agave angustifolia
Martin Hidalgo-Reyes1,3, Magdaleno Caballero-Caballero1, Luis Héctor Hernández-Gómez2 and Guillermo Urriolagoitia-Calderón2
1 Instituto Politécnico Nacional, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional, Unidad Oaxaca, Oaxaca, Mexico.
2 Instituto Politécnico Nacional, Escuela Superior de Ingeniería Mecánica y Eléctrica, Unidad Zacatenco, México, D.F., Mexico.
3 Corresponding author: hogladi@hotmail.com.
Received: September 11th, 2014.
Accepted: December 19th, 2014.
Abstract
The main aim of this study was to characterize cooked bagasse fibers from Agave angustifolia Haw. The fibers were characterized using scanning electron microscopy, differential scanning calorimetry, thermogravimetric analysis, X-ray Diffraction and chemical analysis. The tensile strength was also tested using fibers with a uniform length (30 mm). The fibers were light brown in color, with a mean diameter and length of 501 μm and 144 mm, respectively. Scanning electron microscopy images revealed elliptically shaped cells with varying lumen size. Holocellulose content was approximately 82.12 %, and total lignin content was approximately 20.69 %. Due to the crystallinity and lignin content, the fibers proved to be thermo-stable until 220 °C. The mean values of tensile strength, Young's modulus, % strain (ε), and ultimate tensile strength were determined via mechanical tests. The results are comparable to those of other common lignocellulosic fibers, confirming their potential use as a reinforcing element in a polymer matrix to form a new biodegradable composite.
Keywords: chemical composition, crystallinity, mechanical tests, morphology, thermal analysis.
Resumen
El objetivo principal de este estudio fue caracterizar las fibras de bagazo cocido de Agave angustifolia Haw. Las fibras fueron caracterizadas a través de microscopia electrónica de barrido, calorimetría diferencial de barrido, análisis termogravimétricos, difracción de rayos X y análisis químico. También se realizaron pruebas de resistencia a la tracción usando fibras de longitud constante (30 mm). Las fibras presentaron un color marrón claro, con diámetro medio de 501 μm y longitud media de 144 mm. Las imágenes del microscopio electrónico de barrido mostraron células de forma elíptica con diferente tamaño de lumen. El contenido de holocelulosa fue alrededor de 82.12 % y el contenido total de lignina de aproximadamente 20.69 %. La fibra resultó ser térmicamente estable hasta 220 °C debido a la cristalinidad y el contenido de lignina. El esfuerzo de tensión, el módulo de Young, el porcentaje de deformación (ε) y el esfuerzo último de tensión fueron obtenidos de las pruebas mecánicas. Los resultados son comparables a los de otras fibras lignocelulósicas comunes, lo cual confirma que estas fibras tienen potencial como refuerzo en una matriz polimérica para formar un nuevo compuesto biodegradable.
Palabras clave: análisis térmico, composición química, cristalinidad, morfología, pruebas mecánicas.
Recently, research on plant fibers has been increasing due to the abundance of these materials and their status as renewable resources (Joseph et al., 1999; Ghali et al., 2006; Lucena et al., 2009; Ku et al., 2011; Kestur et al., 2013). Consequently, this focus has led to further research on the specific characterization of individual plant fibers such as bamboo, okra, sisal, and henequen (Mishra et al., 2004; Béakou et al., 2008; De Rosa et al., 2010; Liu et al., 2012; Arrakhiz et al., 2013) and studies on composite materials (Mohanty et al., 2002; Herrera-Franco and Valadez-González, 2005; Faruk et al., 2012). However, fibers from Agave plants have been little studied: of the 256 recorded species, only a handful have been thoroughly analyzed, most notably A. fourcroydes, A. salmiana, A. sisalana, and A. tequilana (Li et al., 2000; Piven et al., 2001; Iñiguez et al., 2005; Velázquez-Jiménez et al., 2013).
In general, information reported regarding the harvesting of the agave, using the by-products and plant parts, and characterizing the plant is very limited. Hence, many areas still need to be investigated. Although studies on Agave angustifolia are scarce (Vera Guzmán et al., 2009; Esqueda et al., 2011; Mejía-Franco and Arzate-Fernández, 2011; Bautista and Smit, 2012; Martinez Gutierrez et al., 2012; Cruz-García et al., 2013; Allsopp et al., 2013), the potential benefits of this lignocellulosic material cannot be ignored. Additional research on its properties is needed so that future alternative uses may be identified and contribute to economic activity by increasing development opportunities, as has occurred with other species (Iñiguez-Covarrubias et al., 2001a; Iñiguez-Covarrubias et al., 2001b; Piven et al., 2001; Gil-Vega et al., 2006; Bessadok et al., 2008; Velazquez-Jimenez et al., 2013).
Cooked Agave angustifolia bagasse is a material with numerous fibers and is a largely underutilized waste material from the mezcal (alcoholic beverage) industry; however, these fibers, similar to other plant fibers, are lignocellulosic materials with technical, economic and ecological potential and therefore could be used in different industries. This agave, known as "maguey espadín," is native to Mexico and is characterized by its spiral-shaped buds that extend radially. Its leaves are rigid, fibrous, linear to lance-shaped and fleshy, with an ascendant horizontal form, pale green to greenish grey in color, flat or rounded at the top, convex at the bottom, narrow and thick toward the base with lateral spines of 2 to 5 mm in length. The stem length ranges from 70 to 90 cm, with mature leaf length ranging from 110 to 130 cm and width from 8 to 10 cm (Chagoya-Méndez, 2004). Commercially, the stem or "piña" is the most important part of the plant because it is the only part used in the production of mezcal, which is the famous traditional alcoholic beverage in Oaxaca, Mexico. The agave is harvested, on average, eight years after being planted. It is then taken to an artisanal production plant, locally referred to as "palenque," where the piñas undergo an artisanal process that involves cutting them into small chunks and then cooking, grinding, fermenting and distilling them. Bagasse is a waste product of this mezcal production process. It is mainly composed of heterogeneous fibers that vary in length and non-fibrous organic material in the form of fine particles (Iñiguez-Covarrubias et al., 2001a). The bagasse is placed in piles (Figure 1) and later moved to agricultural fields for composting.
The mezcal industry in Oaxaca annually produces 122,696 tons of bagasse, an underutilized waste product that is incinerated or dumped into rivers and streams, causing environmental problems (Martínez-Gutiérrez et al., 2013). Its natural degradation causes several problems such as soil pH modification, landscape impairment, odor generation, and an increased concentration of vermin, such as rodents, insects and pathogens; moreover, it is burned in some cases, resulting in environmental damage. However, bagasse fibers are renewable organic matter that have the potential to be used in composite materials to form alternative products and increase the value of the fibers. Because plant fiber characterization is essential to determine bagasse's specific properties and how they are affected by physical, chemical, and anatomical characteristics, the present study aims to characterize Agave angustifolia Haw bagasse fibers to determine their chemical, morphological and mechanical properties. The results reported herein provide information about the properties of this lignocellulosic material to determine potential alternative uses for these fibers. In summary, it is clear (i) that cooked bagasse is a byproduct of the mezcal production process and that its disposal poses an environmental problem, (ii) that the enormous amount of lignocellulose in cooked bagasse has yet to be used to its full potential, and (iii) that reported data on the characteristics and economic potential of these fibers is scarce and widely dispersed.
Materials and methods
Sampling. Cooked bagasse was collected in San Baltazar Chichicapam (W L 96° 29' and N L of 16° 46' at 1,540 m a.s.l.), which is located 56 km south of Oaxaca City, Oaxaca, Mexico (GEO, 2013). A 25 kg amount of bagasse was collected 3 d after distillation, and the quartering method was used. The bagasse was then washed with water to eliminate the remainder of the pith and other impurities. The fibers were manually selected and then dried at ambient conditions (relative humidity: 38 % and temperature: 24 °C) for at least 72 h. Finally, 2.5 kg of fibers were obtained for testing.
Morphology. The fibers' diameter was measured via projection using a Leica DM500 optical microscope with an ICC50 digital camera. The diameter was measured over 100 fibers and measurements were taken at 10 points along the length of each fiber using the LAS EZ software. However, to determine the length of the fiber bundle, a total of 1,000 fibers were measured using a Mitutoyo ruler (No.182-307). For the scanning electron microscopy (SEM) study, the fiber specimens were mounted on cylindrical brass studs with carbon tape and a gold coating was applied using a Fine Coat Ion Sputter JFC 1100 metalizer (JEOL, USA). The fiber samples were observed with a JSM 6390 scanning electron microscope (JEOL, Japan), equipped with an EDAX INCA X-ACT x-ray spectrometer (Oxford Instruments, UK), at 15 keV.
Density. The density was determined from the green volume and the oven-dry weight of the fiber sample. In a vacuum atmosphere, the fibers were saturated with distilled water and the green volume of each sample was obtained using the immersion method. The samples were subsequently dried in a vacuum oven (Mod. 3618, Barnstead International, USA) at 103 ± 2 °C for 8 h. Then, the samples were cooled over silica and weighed again to obtain the weight of the oven-dry sample. The basic density was calculated with equation 1.

where Db is the basic density, P0 is the oven-dry weight of the fiber sample (g) and Vv is the green volume of the fiber sample (cm3).
Chemical characterization. The fibers were ground and sieved, the moisture content was determined, and then the fibers were chemically characterized with the following determinations: soluble products in hot/cold water, solubility in 1 % NaOH solution, percentage of products traceable in an ethanol/toluene solution, the amount of extractables, soluble and insoluble lignin (Klason lignin) content, holocellulose and cellulose content, α-cellulose fraction, and finally, ash content. All of the tests were conducted in triplicate, and the values presented are the mean values.
Ground and sieved. - The fibers were ground with a Willy Mill (Arthur H. Thomas Type). The material was then sifted through sieves (ASTM E11) of different sizes (No. 40 and 60). The ground material passed through the 425 μm sieve but did not pass through the 250 μm sieve. For each test, ~2 g of ground fiber were used.
Moisture content. - The fibers' moisture content was determined using a Mettler Toledo HB43 humidity analyzer with an infrared halogen light and adjusting for standard drying with a response interval of 30 s and a drying temperature of 100 °C (at 2,200 m a.s.l.).
Soluble products in cold/hot water. - The ASTM D1110-84R07 standard was followed to determine the content of soluble products in cold/hot water. The solubility was calculated with equation 2.
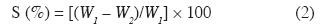
where S is the percentage of soluble products in hot/cold water, W1 is the sample's initial oven-dry weight and W2 is the sample's oven-dry weight after the test.
Solubility in 1 % NaOH solution. - To determine the solubility in 1 % NaOH solution, the TAPPI T212 om-02 standard was followed.
Extractable products in ethanol/toluene solution. - The extractable products in the ethanol/toluene solution were determined in accordance with the ASTM D1107-96R07 standard. To calculate the extractives, an equation similar to equation 2 was used, but in this case, S (%) is the percentage of extractives in ethanol/toluene, W1 is the oven-dry weight of the initial sample and W2 is the oven-dry weight of the sample after ethanol/toluene extraction.
Klason Lignin. - To determine the acid insoluble lignin content, the ASTM D 1106-96 standard was followed. An extractive-free fiber sample was used. The calculations were made using equation 3.
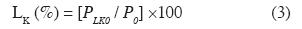
where LK (%) is a percentage of Klason lignin, P0 is the oven-dry weight of the extractive-free fiber sample and PLK0 is the oven-dry weight of the Klason lignin.
Soluble lignin content. - To determine the content of acid soluble lignin, the TAPPI TUM 250 standard was followed. A sample (5 mL) of the hydrolyzed solution, obtained from the determination of the Klason lignin, was analyzed and a UV/vis spectrophotometer was used to measure the absor-bance at 206 nm.
Holocellulose content and the α-cellulose fraction. - The holocellulose content was determined using the sodium chlorite method established by Rowell et al. (2005), and the α-cellulose fraction was determined following the ASTM D1103-60 standard.
Ash content. - The ash content was determined according to the ASTM D1102-84R07 standard and using equation 4.

where A1 is the oven-dry weight of the ash and A2 is the oven-dry weight of the sample.
X-Ray Diffraction (XRD) test. This test was conducted with a Bruker diffractometer, model D8-ADVANCE with Kα1 Cu characteristic radiation and a Linx Eye device to capture diffracted simultaneous radiation at an interval of 3° 2θ with germanium monochromator, radiation (λ= 1,540619 Å), and in operating conditions of 40 keV and 20 mA. The regular diffraction method was used to determine the crystallinity of the fiber. A 100 mm3 sample was used to obtain the best random distribution of the material to estimate the crystallinity index. The measurements were conducted with 1° openings. The crystalline index (CrI) was determined using equation 5 and according to the method proposed by Segal et al. (1959).

where I002 is the intensity of the maximum crystalline spike at 2 θ between 22° and 23°, Iam is the intensity of the minimum crystalline spike at 2 θ between 18° and 19°.
Thermal analyses. To quantify the influence of drying on the agave fiber, thermogravimetric analyses (TGA) were conducted using a TGA Q5000 V3.10 Build 258 machine with a heating range of 20 °C/min. The sample was analyzed in two atmospheres: one with nitrogen and the other with air/oxygen. The experimental conditions were nitrogen (50 mL/min) at an interval of 25 - 500 °C and air (50 mL/min) at an interval of 25 - 700 °C. The differential scanning calorimetry (DSC) test uses the fiber's thermal characteristics as a function of combined moisture and heat treatments to identify transitions and structural modifications of the fiber. A differential scanning calorimeter TA-Instrument (model Q2000) was used in the 50 - 400 °C range with a heating speed of 10 °C/min and in a nitrogen atmosphere with a pressure of 104 Pa.
Tensile properties. Tensile strength testing was performed on individual fibers, considering the mean diameter value (501 μm) that was previously obtained. Fibers 30 mm in length were cut and clamped in a model 3382 universal testing machine (Instron, USA) with a 100 N testing capability load cell and loaded at a constant crosshead displacement rate of 5 mm/min up to failure to obtain the stress-strain graphic. At least 50 samples were tested. The mean values of UTS, YM and ε were obtained from the stress-strain curves. All trials were conducted at an ambient temperature of 22 ± 1 °C and a relative humidity of 38 ± 2 %.
Results
Fiber morphology. The fiber bundles were light brown in color. The histogram in figure 2 shows the frequency distribution of the fiber bundles' mean length. The length values range between 50 and 240 mm and 15 intervals were established. The results reveal a normal distribution with a standard deviation of 2.79 and a standard error of 0.0015. The minimum and maximum length values were 53 and 232 mm, respectively. The highest frequency length was between 136 and 152 mm, and the mean fiber bundle length was 144 mm.
The results corresponding to the fiber bundles' diameter are presented in Table 1. The diameter varies along the fiber bundle. The mean fiber bundle diameter was ~501 μm. For each range, a similar variation trend was noticed with respect to the mean standard deviation. The standard deviations Df1 and Df3 are similar; however, a difference can be observed with respect to Df2.

Micrographs obtained by SEM show the transversal (Figure 3A) and longitudinal (Figure 3B) sections of the cellular structure of Agave angustifolia fiber bundles. The fiber bundle has a type C groove along the shaft and is grouping ~190 - 210 fibers (Figure 3A); they are separated by a middle lamella and are closely grouped. The fibers are oval and polygonal. The cell wall is thick and is composed of a primary and secondary wall, which are closely joined. The mean lumen of each fiber was ~28.6 μm and mean wall thickness ~2.4 μm. The width of the lamella was ~1.7 μm. The width of the wall between two microtubes was ~6.5 μm, and almost at the periphery of the fiber bundle, intercellular spaces such as lumen of some parenchyma cells containing calcium oxalate crystals can be observed. The longitudinal disposition of a large quantity of individual fibers, and the internal structure of the microfibrils can be observed in Figure 3B. This view of the fiber bundle, indicates it is a vascular bundle where a scarce tracheal elements are appreciated. The remaining cells are walls of the fibers and outward from these, the short cells resembles parenchymal cells. The mean length and width of each fiber were ~580.3 μm and ~33.4 μm, respectively. The external longitudinal surface of the fiber bundle is knotty and rough with transversal and longitudinal veins. An irregular grate pattern can be observed due to the grooves. Some damage can also be observed in Figure 3B as broken fiber walls; this is mechanical damage (ripped, scraped, and some fractures) that may be due to the mezcal-making process.
Density. The density determined for this fiber was 414.7 kg/m3. Values have not been previously reported for this species, but the density values reported for the fibers of other species are as follows: 880 kg/m3 for Agave tequilana fibers (Kestur et al., 2013), 740 kg/m3 for A. americana fibers (Bessadok et al., 2008) and 1450 kg/m3 for A. sisalana fibers (Li et al., 2000).
Chemical Composition. Table 2 summarizes the fiber's chemical analysis. It can be observed that the moisture content obtained (7.78 %) is lower than that previously reported (10.1 %) by Kestur et al. (2013). A similar situation was observed in almost all of the chemical analyses; for example, the solubility percentage values in cold/hot water (2.66 % / 4.39 %) are lower than values (not reported / 5.84 %) documented by Iñiguez et al. (2005) and values (9.74 % / 10.64 %) reported by Kestur et al. (2013), which were both from cooked Agave tequilana material. However, the solubility value in the 1 % NaOH solution obtained in this study was 20.22 %, although in accordance with the TAPPI T212 om-02 standard, acceptable values are considered to be between 11.2 % and 17.0 %. In the case of the ethanol/toluene extracts, this value (1.54 %) is lower than the 2.95 % and 3.1 % values reported for cooked A. tequilana material by Kestur et al. (2013) and Iñiguez et al. (2005), respectively.
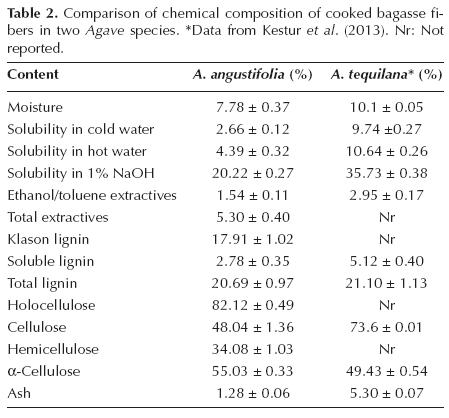
Similarly, the obtained value of Klason lignin (17.91 %) is higher than that (7.2 %) reported by Iñiguez et al. (2005), whereas the obtained value of soluble lignin (2.78 %) is lower than that (5.12 %) reported by Kestur et al. (2013). Thus, some degree of similarity can be observed in total lignin content between the obtained value (20.69 %) and that previously (21.1 %) reported by Kestur et al. (2013); however, the total lignin content is higher than the values for raw fiber (16.8 %) and cooked fiber (7.2 %) reported by Iñiguez-Covarrubias et al. (2001b) and reported by Iñiguez et al. (2005), respectively. In relation to the α-cellulose content, the value obtained (55.03 %) is higher than that reported (49.43 %) by Kestur et al. (2013) and also higher than that reported (41.90 %) by Iñiguez et al. (2005) but is lower than that reported (64.9 %) when raw material was used (Iñiguez-Covarrubias et al., 2001a).
Finally, the ash content value (1.28 %) is lower than the values (5.30 % and 8.8 %) reported by Kestur et al. (2013) and Iñiguez et al. (2005), which were both from cooked Agave tequilana material.
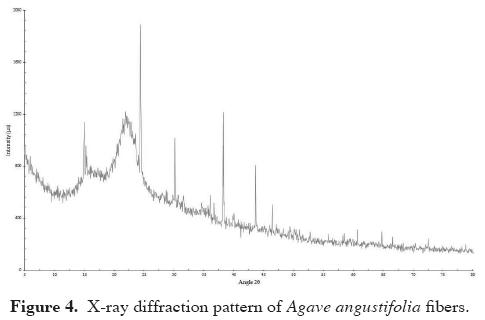
X-ray diffraction analysis (XRD). Figure 4 shows the x-ray diffraction pattern of Agave angustifolia fibers. Various and repeated sharp peaks can be observed, indicating a crystalline phase. The diffraction pattern displays peaks corresponding to A-type amylose allomorph. This is characterized by a large spike at 2 θ = 16.5° and a very large spike at 2 θ = 21.9°, which corresponds to the organic phase and the consequence of the specific reflection of their crystalline structure. The crystallographic information on the phases presented by the analyzed fibers, in relation to the qualitative analysis, shows that the crystalline phase corresponds to the compound calcium oxalate hydrate (CaC2O4 (H2O)) with a monoclinic crystalline system. The identification of the inorganic crystal structure database is 030782 and the values obtained from this analysis are as follows: space group: P 21/c; a = 6.29 Å; b = 14.58 Å; c = 10.11 Å; α = γ = 90°; β = 109.46°; V = 874.9 Å3.
Thermal Studies. Figure 5 shows the graphics in two atmospheres: nitrogen and air, respectively. The lost fiber weight at three different temperatures: ~7.8 % at 80 °C (fiber's humidity content), 59.8 % at 298 °C, and finally, 22 % at 618 °C. Between 80 and 225 °C, the sample was thermally stable, which suggests that these fibers are safe to use up to a maximum temperature of 225 °C. The lost sample weight showed temperatures greater than 220 °C and up to 620 °C. Moreover, a residue of approximately ~5 % can be observed in Figure 5A and is most likely of a mineral nature.
The free water content was measured by the vaporization temperatures and obtained with the DSC study. In this case, the fibers present (Figure 5C) two endothermic peaks, one at 147.37 °C and the other at 203.83 °C, which correspond to fiber dehydration and cellulose decomposition, respectively, and the quantity of energy used to evaporate the water was ΔH = 3.8 J/g and ΔH = 18.56 J/g, respectively.
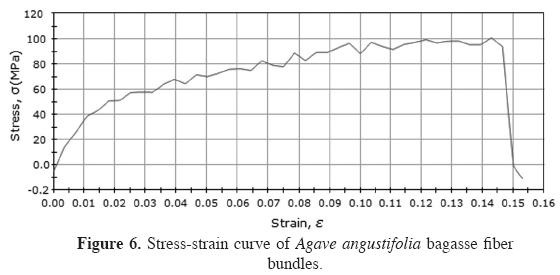
Tensile properties. Figure 6 shows the typical stress-strain curve of Agave angustifolia fibers. The principal tensile properties were evaluated using these curves. The mean UTS value (86.9 MPa) presented a standard deviation of 0.314; the mean YM value (2.509 MPa) presented a standard deviation of 0.721; and the mean 8 value (14.187) showed a standard deviation of 5.414. This property does not differ significantly with the diameter at the 0.05 level. However, the mean TS value obtained in this study was 44.167 MPa.
Discussion
All of the study results take into account that the field of lignocellulosic fibers is very broad and that their properties depend to a great extent on factors such as climate variation, plant age, experimental processes, and conditions during testing (John and Thomas, 2008).
Fiber morphology. In this case, the fiber bundles have a similar morphology to that of other plant fibers, such as Agave sisalana (Alves-Fidelis et al., 2013) and A. tequilana (Kestur et al., 2013) because all of them are composed of many fiber cells. Each fiber cell is united by the middle lamella, which consists of cellulose and lignin, but they differ by factors such as the number of fiber cells, the cell-wall size, the thickness of the secondary cell-walls, the real cross-section (the total area minus the lumen area), and the fiber bundle cross-section area. According to Alves-Fidelis et al. (2013), it is possible to correlate fiber morphology with tensile strength properties. Thus, each fiber bundle presents its own specific morphological characteristics and therefore its respective mechanical behavior. Fiber strength can be an important factor in selecting a specific natural fiber for a particular application, and changes in physical properties can be due to differences in fiber morphology. Major differences in structure, such as density and cell wall thickness, length and diameter, result in differences in physical properties (Faruk et al., 2012).
Knowledge of fiber bundle length and width (diameter) is very important because it provides an indication of the possible strength properties of different types of natural fibers (Alves-Fidelis et al., 2013). In this study, the fiber bundles show a wide variability in length, which corresponds to the standard deviation value (2.79). The mean length (144 mm) of the fiber bundle is longer than that reported (110 mm) by Kestur et al. (2013) for Agave tequilana. It could be due to factors such as the variety, maturity, and the analyzed part of the plant, but it also largely depends on the material extraction process. In this case, the process is purely manual; "piñas" are cut completely at random using machetes and axes, whereas other materials, such as A. tequilana, are processed in an industrial way (machine cut), and therefore, the differences between the results and those reported previously are understandable. A different situation occurs with respect to the diameter, despite varying along the fiber bundle, but the fiber extraction process does not have any influence on this variability. In addition, the mean diameter (501 /m) obtained is less than that reported (592.34 μm) by Kestur et al. (2013) for A. tequilana fiber bundles, but this variability could be due to irregular fiber growth as a result of plant growing conditions such as climate and soil type, harvest age, and plant species.
Density. It is important to know the different properties of each natural fiber, such as density, to be able to better exploit its potential. In this case, the obtained density value (414.7 kg/m3) is lower than that reported for other species of Agave (Li et al., 2000; Bessadok et al., 2008; Kestur et al., 2013). This may be due to differences between the species but also to the treatment of the fiber during the mezcal production process. In this sense, it should be noted that the bagasse used in this study was subjected to physical and chemical conditions that caused a natural decomposition process that can also influence density. Biocomposites reinforced with natural fibers have been developed over the past several years because of their significant processing advantages, biodegradability, low cost, renewable nature, high specific strength, and low relative density (Faruk et al., 2012). In addition, natural fibers are suitable for reinforcing polymeric matrix composites due to the characteristics mentioned above.
Chemical Composition. In addition to morphology, chemical composition can also have a bearing on the properties of natural fibers (Cedeño-Cruz and Alvarez-Jacobs, 1999). Due to the variety and reported data, the main comparison of this analysis was made with data from Agave tequilana previously reported by Kestur et al. (2013). Although they have almost the same physical characteristics and both are from the same genus, their chemical constituents show differences as observed in the results section. Hot water was used to extract tannins, rubber, sugar, starch, and colored materials. Generally, natural resins are thermally stable and insoluble, which could influence solubility in hot water values for the current study and in previous works. The solubility percentage in hot water (4.39 %) is lower than the values (5.84 % and 10.64 %) reported by Iñiguez et al. (2005) and Kestur et al. (2013) for cooked Agave tequilana bagasse fibers. This may be because the processes are different. Agave angustifolia fibers are cooked, fermented, and distillated, which is an aggressive treatment, but A. tequilana fibers do not undergo a distillation process because they are only cooked, fermented, and squeezed.
In this study, the solubility value (20.22 %) obtained in the 1 % NaOH solution is higher than the acceptable value range (from 11.2 to 17.0 %) established in the TAPPI T212 om-02 standard. Therefore, the value obtained suggests that the fibers were partially degraded, due to fermentation and distillation. This is most likely because the analyzed fibers were obtained from the waste of the mezcal production process. However, the value obtained is lower than that (35.73 %) reported by Kestur et al. (2013), which could be due to the amount of time the bagasse is in the waste pile prior to being collected. As long as the material remains in the waste pile, its degradation process never stops. In this case, cooked bagasse was collected 3 d after distillation and tested 30 d thereafter.
Lignocellulosic material was analyzed to determine the extent of fungal attacks and the effect of other harmful agents, such as heat, light, and oxidation, among others. These data are important due to the natural humidity of this waste product and its high probability of deterioration. Raw Agave fibers impede decomposition, but cooked bagasse fibers can show signs of decomposition prior to testing. Thus, the time period prior to testing is a factor that affects the fibers' chemical composition. These tests were conducted 30 d after collection; fibers (clean and dry) were stored at a temperature of 23 ±1 °C and a relative humidity of 38 %. Therefore, fiber degradation as a result of fungi or bacteria is not likely to have occurred during this period.
The main component of fiber resistance is cellulose. Sisal fiber has a cellulose content of approximately 73 % (Sydenstricker et al., 2003), Agave tequilana has a cellulose content of 73.6 % (Kestur et al., 2013), jute has a cellulose content of 65 % (Wang et al., 2008), and the studied fiber has a cellulose content of 48 %. Therefore, A. tequilana and sisal fibers may have higher resistance, not only because of their morphological characteristics but also because of their higher cellulose content. The total of the chemical components (cellulose + lignin + ash) is ~104 %. In theory, the sum of all of the components identified using chemical analysis for lignocellulosic materials should be 100 %; however, this value may vary due to the superposition and partial loss of some components such as the failure to consider the residues present in the material (Browning, 1967). Differences between the obtained data and previously reported data for the agave fibers of other species could be due to a variety of factors, including geographical location, harvesting, plant maturity, fiber extraction process, and applied methodology to identify properties (Iñiguez et al., 2005; Parra-Negrete et al., 2010; Kestur et al., 2013).
X-ray diffraction analysis (XRD). The crystalline index obtained for this fiber was 43 %. It had a unique diffraction pattern that is typical in cellulose fibers. Some points suggest a highly organized crystalline material, most likely due to the concentration of the internal microcrystalline impurities observed in the fiber (Figure 3A). This confirms the sharp peaks observed in the diffractogram. The results of the x-ray diffraction study show various flat peaks, which can be associated with the crystalline character of the fiber and also due to a type of sugar or another substance added during the fermentation process (Tronc et al., 2007). In this case, WinPLOTR software was used to trace and visualize the diffractogram corresponding to the diffraction profiles of the crystalline part, the dispersion of the amorphous part and data processing. The diffraction database provided by the Inorganic Crystal Structure Database was used to identify the phases.
Thermal Studies. One of the limiting factors in the use of natural fibers as a reinforcement in biocomposites is their low thermal stability. For this reason, the thermal stability of cooked bagasse fibers was determined using thermogravimetric analysis (TGA), which provides information about the thermal stability of the fibers and their composition in terms of volatile substances, organic compounds and inorganic residuals. The thermal studies were conducted in two atmospheres, nitrogen and air. The first atmosphere impeded combustion and allowed the decomposition of the components one by one. It is therefore possible to identify the decomposition regions of the fiber's components. In the second atmosphere, the reactions occurred simultaneously, and it is therefore not possible to separate the different decomposition processes of the fiber's components such as hemicellulose, cellulose and lignin (Tomczak et al., 2007). In this study, the weight loss that occurred at temperatures higher than 220 °C and up to 620 °C may be attributed to the decomposition of the different cell types within the fiber. The above weight losses can be attributed to the oxidation of the fragments upon exposure to air. The sample was thermally stable, which suggests that these fibers are safe to use up to a maximum temperature of 225 °C. This temperature can be considered a thermal stability value for this fiber and is directly related to the high crystallinity values (43.0 %) and the high lignin content (20.69 %). Therefore, it can be concluded that the TGA curves indicate the fiber's humidity content, thermal stability and ash content.
The 7.8 % water loss observed in the TGA curve is water recuperated as capillary water, which can be related to ambient humidity and temperature throughout the fiber. In fact, the value corresponds to the water content (7.8 %) and is calculated as the difference between the weight of the fiber before and after drying at a constant temperature. It must be noted that even though the fibers were dried prior to testing, it is difficult to completely eliminate the fiber's water content due to its hydrophilic nature. Therefore, this water is recuperated by other components of the fiber (soluble and insoluble lignin, along with small quantities of amorphous cellulose). This is due to the fiber's high crystallinity.
It has been reported (Princi et al., 2005) that the best estimation of the free water content in cellulosic materials is obtained using vaporization temperatures with DSC. In this case, the fibers show two endothermic peaks (at 147.37 and 203.83 °C), corresponding to fiber dehydration and cellulose decomposition, respectively. This is in contrast with Agave tequilana, where only one peak was observed at 89 °C (Kestur et al., 2013) corresponding to fiber dehydration. The comparison of these results with those recently obtained for A. tequilana by Kestur et al. (2013) could be useful as the two fibers are chemically similar, although they differ in water content. In addition, they have different weight losses at similar temperatures, although such a comparison is not suitable due to differences in experimental conditions. Kestur et al. (2013) reported a spike and massive sample losses of 10.8 % at 80 °C, 63.8 % at 360 °C, and 20 % at 620 °C, which were attributed to water elimination and cellulose and lignin degradation, respectively. Consequently, the findings of this study can be similarly understood, in relation to previously reported results for lignocellulosic materials, due to water elimination and cellulose and lignin degradation.
Tensile properties. The stress-strain curve (Figure 6) shows the typical behavior of a viscoelastic material, which is understandable due to the nature of this lignocellulosic fiber. A continual stress increase was observed until a maximum strain was reached, at which point the fiber failed. In this case, the curve obtained was similar to those reported for similar fibers such as those for Agave tequilana mentioned by Kestur et al. (2013). The values found for YM and TS show that these properties differ significantly with diameter at the 0.05 level. The values obtained present variability, as do the results of other natural fibers analyzed in previous studies. In this study, the fibers with the largest diameter had higher values, in terms of mechanical properties, and the fibers with the smallest diameter had lower values. The fiber's elongation demonstrates that it is a fragile material, and thus, its characteristics are similar to those of most reinforcements for composite materials. Theoretically, this fiber stands out as an excellent substitute for natural and even synthetic fibers for applications in which criteria such as low cost, recyclability, weight, low density, and high specific strength are relevant.
In conclusion, the agave bagasse is a waste material from the mezcal process and has an abundant availability; therefore, knowledge of the properties of these cooked fibers provides alternatives, not only as a value addition to these residues but also to generate job opportunities in the particular region where the species grows. In addition the bagasse fibers of Agave angustifolia have the potential to be used in diverse applications because they are comparable with those reported for other common lignocellulosic fibers. Due to the fibers' thermal properties, low density, and high cellulose content, a future application could be their incorporation into a polymeric matrix to produce potential composite materials.
Acknowledgements
The senior author would like to thank the Universidad Autónoma Chapingo and particularly Marcos González Peña, Director of the Wood Technology Laboratory, for his invaluable help over the course of this research. Thanks are also extended to Greta Rosas Saito (Colegio de Postgraduados) for SEM technical assistance, to Yolanda Ortiz Hernández (CIIDIR-OAXACA) for her advice and criticisms of the manuscript, and two anonymous reviewers which help to clarify some ideas.
Literature cited
Allsopp P., Possemiers S., Campbell D., Oyarzábal I.S., Gill C. and Rowland I. 2013. An exploratory study into the putative prebiotic activity of fructans isolated from Agave angustifolia and the associated anticancer activity. Anaerobe 22:38-44. [ Links ]
Alves-Fidelis M.E., Castro-Pereira T.V., Martins-Gomes O.F., de Andrade-Silva F. and Toledo-Filho R.D. 2013. The effect of fiber morphology on the tensile strength of natural fibers. Journal of Materials Research and Technology 2:149-157. [ Links ]
Arrakhiz F.Z., El Achaby M., Malha M., Bensalah M.O., Fassi-Fehri O., Bouhfid R., Benmoussa K. and Qaiss A. 2013. Mechanical and thermal properties of natural fibers reinforced polymer composites: Doum/low density polyethylene. Materials and Design 43:200-205. [ Links ]
Arzate-Fernández A.M. and Mejía-Franco R. 2011. Capacidad embriogénica de callos inducidos en ejes embrionarios cigóticos de Agave angustifolia Haw. Revista Fitotecnia Mexicana 34:101-106. [ Links ]
Bautista J.A. and Smit M.A. 2012. Sustentabilidad y agricultura en la "región del mezcal" de Oaxaca. Revista Mexicana Ciencias Agrícolas 3:5-20. [ Links ]
Béakou A., Ntenga R., Lepetit J., Atéba J.A. and Ayina L.O. 2008. Physico-chemical and microstructural characterization of "Rhectophyllum camerunense" plant fiber. Composites Part A: Applied Science and Manufacturing 39:67-74. [ Links ]
Bessadok A., Marais S., Roudesli S., Lixon C. and Métayer M. 2008. Influence of chemical modifications on water-sorption and mechanical properties of Agave fibres. Composites Part A: Applied Science and Manufacturing 39:29-45. [ Links ]
Browning BL. 1967. Methods of Wood Chemistry, Vol. 1, John Wiley & Sons, Incorporated, New York. [ Links ]
Cedeño-Cruz M. and Alvarez-Jacobs J. 1999. Production of tequila from agave: historical influences and contemporary processes. In: Jaques K.A., Lyons T.P. and Kelsall D.R. Ed. The Alcohol Textbook: A Reference for the Beverage, Fuel and Industrial Alcohol Industries, pp. 223-245. Nottingham University Press, Nottingham. [ Links ]
Chagoya-Méndez V.M., 2004. Diagnóstico de la cadena productiva del sistema producto maguey-mezcal. Consejo Oaxaqueño del maguey y mezcal A.C. Secretaría de Agricultura Ganadería, Desarrollo Rural Pesca y Alimentación (SAGARPA). Delegación Oaxaca, Oaxaca, México. 167 p. <http://www.amsda.com.mx/PREstatales/Estatales/OAXACA/PREmezcal.pdf> (accessed 20.06.15). [ Links ]
Cruz-García H., Enríquez-del Valle J.R., Velasco-Velasco V.A., Ruiz-Luna J., Campos-Ángeles G.V. and Aquino-García D.E. 2013. Nutrimentos y carbohidratos en plantas de Agave angustifolia Haw y Agave karwinskii Zucc. Revista Mexicana de Ciencias Agrícolas Especial 6:1161-1173. [ Links ]
De Rosa I.M., Kenny J.M., Puglia D., Santulli C. and Sarasini F. 2010. Morphological, thermal and mechanical characterization of okra (Abelmoschus esculentus) fibres as potential reinforcement in polymer composites. Composites Science and Technology 70:116-122. [ Links ]
Esqueda M., Gutiérrez A., Palomino G., García-Mendoza A. and Terrazas T. 2011. Morphological characterization and variation in the total content of reducing sugars in wild populations of Agave angustifolia Haw. American Journal of Agricultural and Biological Sciences 6:462-468. [ Links ]
Faruk O., Bledzki A.K., Fink H.P. and Sain M. 2012. Biocomposites reinforced with natural fibers: 2000-2010. Progress in Polymer Science 37:1552-1596. [ Links ]
GEO [Gobierno del Estado de Oaxaca], 2013. Enciclopedia de los Municipios y Delegaciones de México. Ayuntamiento de San Baltazar Chichicapam. Gobierno del Estado de Oaxaca. < http://www.inafed.gob.mx/work/enciclopedia/EMM20oaxaca/municipios/20112a.html > (accessed 25.08.15). [ Links ]
Ghali L., Zidi M. and Roudesli S. 2006. Physical and mechanical characterization of technical esparto (alfa) fibres. Journal of Applied Science 6:2450-2455. [ Links ]
Gil-Vega K., Díaz C., Nava-Cedillo A. and Simpson J. 2006. AFLP analysis of Agave tequilana varieties. Plant Science 170:904-909. [ Links ]
Herrera-Franco P.J. and Valadez-González A. 2005. A study of the mechanical properties of short natural-fiber reinforced composites. Composites Part B: Engineering 36:597-608. [ Links ]
Iñiguez-Covarrubias G., Lange S.E. and Rowell R.M. 2001a. Utilization of byproducts from the tequila industry: Part 1: agave bagasse as a raw material for animal feeding and fiberboard production. Bioresource Technology 77:25-32. [ Links ]
Iñiguez-Covarrubias G., Díaz-Teres R., Sanjuan-Dueñas R., Anzaldo-Hernández J. and Rowell R.M. 2001b. Utilization of by-products from the tequila industry. Part 2: potential value of Agave tequilana Weber azul leaves. Bioresource Technology 77:101-108. [ Links ]
Íñiguez G., Acosta N., Martínez L., Parra J. and González O. 2005. Utilización de subproductos en la industria tequilera. Parte 7. Compostaje de bagazo de agave y vinazas tequileras. Revista Internacional de Contaminación Ambiental 21:37-50. [ Links ]
John M.J. and Thomas S. 2008. Biofibres and biocomposites. Carbohydrate Polymers 71:343-364. [ Links ]
Joseph K., Tolêdo-Filho R.D., James B., Thomas S. and Hecker-de Carvalho L. 1999. A review on sisal fiber reinforced polymer composites. Revista Brasileira Engenharia Agrícola e Ambiental 3:367-379. [ Links ]
Kestur G. S., Flores-Sahagun T.H.S., Dos Santos L.P., Dos Santos J., Mazzaro I. and Mikowski A. 2013. Characterization of blue agave bagasse fibers of Mexico. Composites: Part A. 45:153-161. [ Links ]
Ku H., Wang H., Pattarachaiyakoop N. and Trada M. 2011. A review on the tensile properties of natural fiber reinforced polymer composites. Composites Part B: Engineering 42:856-873. [ Links ]
Li Y., Mai Y.W. and Ye L. 2000. Sisal fibre and its composites: a review of recent developments. Composites Science Technology 60:2037-2055. [ Links ]
Liu D., Song J., Anderson D.P., Chang P.R. and Hua Y. 2012. Bamboo fiber and its reinforced composites: structure and properties. Cellulose 19:1449-1480. [ Links ]
Lucena M.P., Suarez A. and Zamudio I. 2009. Desarrollo de un material compuesto a base de fibras de bambú para aplicaciones aeronáuticas. Suplemento de la Revista Latinoamericana de Metalurgia y Materiales S1:1107-1114.
Martínez-Gutiérrez G.A., Íñiguez-Covarrubias G., Ortíz-Hernández Y.D., López-Cruz J.Y. and Bautista-Cruz M.A. 2013. Tiempos de apilado del bagazo del maguey mezcalero y su efecto en las propiedades del compost para sustrato de tomate. Revista Internacional de Contaminación Ambiental 29:209-216. [ Links ]
Martínez-Gutiérrez G.A., Zárate-Altamirano G. and Urrestarazu M. 2012. Maguey bagasse waste as sustainable substrate in soilless culture by melon and tomato crop. Journal of Plant Nutrition 35: 2135-2144. [ Links ]
Mishra S., Mohanty A.K., Drzal L.T., Misra M. and Hinrichsen G. 2004. A review on pineapple leaf fibers, sisal fibers and their biocomposites. Macromolecular Materials and Engineering 289:955-974. [ Links ]
Mohanty A.K., Misra M. and Drzal L.T. 2002. Sustainable biocomposites from renewable resources: opportunities and challenges in the green materials world. Journal of Polymers and the Environment 10:19-26. [ Links ]
Parra-Negrete L.A., del Villar-Quiñones P. and Prieto-Rodríguez A. 2010. Extracción de fibras de agave para elaborar papel y artesanías. Acta universitaria 20Esp.3:77-83. [ Links ]
Piven N.M., Barredo-Pool F.A., Borges-Argáez I.C., Herrera-Alamillo M.A., Mayo-Mosqueda A., Herrera-Herrera J.L. and Robert M.L. 2001. Reproductive biology of henequen (Agave fourcroydes) and its wild ancestor Agave angustifolia (Agavaceae). I. Gametophyte development. American Journal of Botany 88:1966-1976. [ Links ]
Princi E., Vicini S., Pedemonte E., Arrighi V. and McEwen I. 2005. Thermal characterization of cellulose based materials: investigation of water content. Journal of Thermal Analysis and Calorimetry 80:369-373. [ Links ]
Rowell R.M., Pettersen R., Han J.S., Rowell J.S. and Tshabalala M.A. 2005. Cell wall chemistry. In: Rowell R.M. Ed. Handbook of Chemistry and Wood Composites, pp. 62-63, Taylor & Francis Group, Boca Raton. [ Links ]
Segal L., Creely J.J., Martin A.E. and Conrad C.M. 1959. An empirical method for estimating the degree of crystallinity of native cellulose using X-ray diffractometer. Textiles Research Journal 29:786-794. [ Links ]
Sydenstricker T.H.D., Mochnaz S. and Amico S.C. 2003. Pull-out and other evaluations in sisal-reinforced polyester biocomposites. Polymer Testing 22:375-380. [ Links ]
Tomczak F., Sydenstricker T.H.D. and Satyanarayana K.G. 2007. Studies on lignocellulosic fibers of Brazil. Part II: Morphology and properties of Brazilian coconut fibers. Composites Part A: Applied Science and Manufacturing 38:1710-1721. [ Links ]
Tronc E., Hernández-Escobar C.A., Ibarra-Gómez R., Estrada-Monje A., Navarrete-Bolaños J. and Zaragoza-Contreras E.A. 2007. Blue agave fiber esterification for the reinforcement of thermoplastic composites. Carbohydrate Polymers 67:245-255. [ Links ]
Velázquez-Jiménez L.H., Pavlick A. and Rangel-Mendez J.R. 2013. Chemical characterization of raw and treated agave bagasse and its potential as adsorbent of metal cations from water. Industrial Crops and Products 43:200-206. [ Links ]
Vera-Guzmán A.M., Santiago-García P.A. and López M.G. 2009. Compuestos volátiles aromáticos generados durante la elaboración de mezcal de Agave angustifolia y Agave potatorum. Revista Fitotecnia Mexicana 32:273-279. [ Links ]
Wang W., Cai Z. and Yu J. 2008. Study on the chemical modification process of jute fiber. Journal of Engineered Fibers and Fabrics 3:1-11. [ Links ]