Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Botanical Sciences
versión On-line ISSN 2007-4476versión impresa ISSN 2007-4298
Bot. sci vol.93 no.4 México dic. 2015
https://doi.org/10.17129/botsci.214
Botánica estructural
Phenotypic variation of flowering and vegetative morphological traits along the distribution for the endemic species Yucca capensis (Agavaceae)
Variación fenotípica de la floración y de caracteres morfológicos vegetativos a lo largo de la distribución de la especie endémica Yucca capensis (Agavaceae)
Maria Clara Arteaga1,4,5, Rafael Bello-Bedoy2,5 , Jose Luis León de la Luz3, José Delgadillo2 y Reymundo Dominguez3
1 Departamento de Biología de la Conservación, Centro de Investigación Científica y de Educación Superior de Ensenada (CICESE), Ensenada, Baja California, México.
2 Facultad de Ciencias, Universidad Autónoma de Baja California, Ensenada, Baja California, México.
3 Centro de Investigaciones Biológicas del Noroeste (CIBNOR), La Paz, Baja California Sur, México.
4 Author for correspondence: arteaga@cicese.mx.
5 These authors contributed equally to this work.
Received: August 13th, 2014.
Accepted : October 11th, 2014.
Abstract
Phenotypic variation across the geographic range of a species depends upon genetic differences within and between populations as well as environmental heterogeneity. Estimating the variation in morphological and reproductive traits and determining the influence of abiotic factors on the expression of phenotype is particularly important in endemic species as a means of inferring their response to different environmental scenarios. This study analyzes the interpopulation variation in floral and vegetative traits of Yucca capensis Lenz and their relation to altitude and precipitation. At 12 different sites total plant length, stem length, stem circumference, rosette length, rosette diameter, leaf length and leaf width were measured and the number of plants with inflorescences and fruits was recorded. The results showed higher coefficients of variation for plant length, stem length and rosette length and lower coefficients of variation for leaf length and width. All of the vegetative traits differed significantly between sites. It was found that 31 % and 12 % of the plants produced inflorescences and fruits respectively and inflorescence production differed between sites, presenting a positive relation with average annual precipitation. This study shows that there is large phenotypic variation in vegetative traits and that levels of rainfall have a clear influence on the production of reproductive structures throughout the geographic range of the endemic species Y. capensis.
Key words: Baja California Sur, inflorescence, fruits, rainfall, environmental variation.
Resumen
La variación fenotípica en la distribución geográfica de una especie depende de diferencias genéticas dentro y entre las poblaciones, así como de la heterogeneidad ambiental. Estimar la variación de caracteres morfológicos y reproductivos y determinar la influencia de factores abióticos sobre la expresión del fenotipo es particularmente importante en especies endémicas para inferir su potencial de respuesta ante diferentes escenarios ambientales. En este estudio analizamos la variación inter-poblacional de caracteres vegetativos y de la floración de Yucca capensis Lenz, así como su relación con la precipitación y la altitud. En 12 localidades medimos la longitud total de la planta, longitud y circunferencia del tallo, longitud y diámetro de roseta, longitud y ancho de hoja y registramos el número de plantas con inflorescencias y frutos. Los resultados mostraron coeficientes de variación mayores para la longitud de la planta, del tallo y de la roseta y menores para la longitud y el ancho de hoja. Además, todos los caracteres vegetativos difirieron significativamente entre localidades. Encontramos que un 31 % y 12 % de las plantas produjeron inflorescencias y frutos, respectivamente y la producción de inflorescencias difirió entre localidades, presentando una relación positiva con la precipitación anual promedio. En este estudio se demostró que hay una gran variación fenotípica de caracteres vegetativos y una influencia clara de la cantidad de lluvia sobre la producción de estructuras reproductivas a lo largo de la distribución geográfica de la especie endémica Y. capensis.
Palabras clave: Baja California Sur, inflorescencia, frutos, lluvia, variación ambiental.
Phenotypic variation shown among individuals across the geographic range of a species can be a result of genetic differences and the influence of environmental factors. Rainfall levels, environmental temperature and availability of nutrients can drastically influence the geographic structure of genetic and phenotypic variation between plant populations (Lamberts and Porter, 1992). For example, among sites with differing levels of light and/or rainfall it has been reported that the variability in plant architecture, growth and reproduction represents different responses specific to particular environments (Bustamente and Burquez, 2008). Documenting spatial variation in vegetative and reproductive phenotypes, and the potential effect of the environment on this variation, will allow us to understand with more detail the ecology and evolutionary history of species.
This study analyzes the variation in flowering and vegetative traits of Yucca capensis L.W. Lenz across its range and the effects of abiotic variables on phenotypic variation. Yucca capensis is endemic to the Cape Region of Baja California Sur, distributed in the undergrowth of the lowland deciduous forest of the mountains, up to 1,000 m a.s.l. (Leon de la Luz et al., 2012), with scattered individuals present within the oak forest (Lenz, 1998). Populations consist of groups of less than 15 individuals and each individual can develop multiple trunks as a result of vegetative growth (Lenz, 1998). Sexual reproduction of Y. capensis, as with the rest of the species of this genus, involves a close mutually beneficial interaction with moths of the family Prodoxidae (Pellmyr et al., 2008); the result of a long coevolutionary process (Baker, 1986).
Until the mid-1990s, populations of Yucca capensis were considered isolated aggregations of Yucca valida Brandegee, a species native to the center-south region of Baja California with an irregular distribution throughout the Pacific coast of the Peninsula (Turner et al., 1995). The Yucca populations on the mountain slopes of Los Cabos show shrub like growth, with a tendency to take the prostrate form, whereas Y. valida display an erect growth form. As well as the difference in prostrate growth, other distinctions in vegetative morphological traits such as leaf size and width resulted in proposing Y. capensis as a new taxonomic entity on the species level (Lenz, 1998). A phylogenetic analysis of the Yucca genus supports the hypothesis that Y. capensis and Y. valida are sister species (Pellmyr et al., 2007). Both species have coevolved with the same pollinator species of moth, Tegeticula baja (Pellmyr et al., 2008).
The restricted distribution of Yucca capensis in the Cape region has been emphasized in assessments of areas of endemism in Mexico (Sosa and de Nova, 2012). However, since its description, there have been no studies focusing on this species, leaving a gap in the knowledge of its biology and ecology. Information about vegetative and reproductive traits is limited to that reported in the descriptive diagnosis (Lenz, 1998), which does not specify how many individuals were considered to generate the data, nor their precise location.
Despite Yucca capensis not being a dominant element in terms of abundance throughout its geographic range (León de la Luz and Breceda, 2006), its ecological function as part of the close mutualistic interaction with the pollinating moth is fundamental, as the permanence of one species depends on the reproductive success of the other. However, part of the range of Y. capensis is suffering from a conversion of land use for touristic and agricultural developments as well as the general expansion of the human population, which could imply a risk for the permanence of both species. This highlights the need to implement studies which will increase biological knowledge of this species and therefore provide the necessary information for the initiation of conservation measures.
Material and Methods
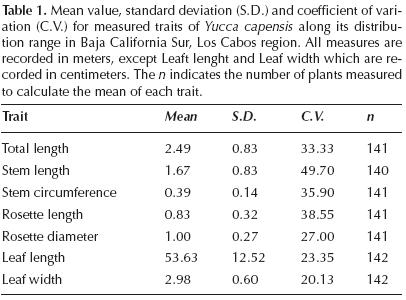
In order to examine vegetative phenotypic variation, the presence of flowers and fruits, and their relation to precipitation and altitude, 12 sites throughout the geographic range of Yucca capensis were sampled. These sites were located between 295 and 789 m a.s.l., in the lowlands of the mountainous areas of Los Cabos (Figure 1) and they were visited between September and December 2013. Measurements of 7 vegetative traits were taken from the individual with the longest trunk in each clonal group, in a total of 142 individuals: (i) total length, from ground to the highest point of the rosette; (ii) stem length, from ground to the start of the rosette; (iii) stem circumference, taken approximately one meter above the start of the stem; (iv) rosette length, from the start of the rosette to its highest point; (v) maximum diameter of the rosette, taken as the distance between the tips of the most horizontally extended leaves; (vi) leaf length, from the base of the apex of a new leaf; (vii) leaf width, taken at the centre of the leaf. Prostrate or erect form was also noted, as well as the presence/absence of flowers or fruits on each tree.
ANOVA was used to assess the presence of significant differences in the morphological measurements between populations for continuous size traits, and logistic ANCOVA was used for the binomial trait, presence of inflorescence (Whitlock y Schluther, 2008). To control the influence of plant size on reproductive variation, plant length was included in the ANCOVA. To estimate the effects of the abiotic factors precipitation and altitude, as well as latitude on flowering, a regresion was used for each of the independent variables. The statistical analyses were carried out using JMP 5.01 (SAS Cary, New Jersey, USA). For each site altitude and latitude data was obtained using a GPS and annual precipitation values were taken from the bioclimatic layer Bio12, downloaded from the official Worldclim website (http://www.worldclim.org), using ArcGis 9.3 software (ESRI, 2009).
Results
The mean number of individuals of Yucca capensis in the 12 sampled sites was 11.83 (s.d. 4.3). In 50 % of the sites, the mean length of the main stem of each individual was less than 2.5 m, this being the trait which showed most variation (Table 1, Figure 2). For example, between sites 11 and 8, the mean maximum and minimum stem lengths were recorded respectively, with a difference of 40 % between the two values (Figure 2). In the rosette, the traits showing least variation were leaf length and width, while the rosette length and diameter presented intermediate values (Table 1; Figure 2). All of the vegetative traits that were measured differed significantly between sites, even controlling for variation due to plant size (Table 2). Finally, 59.4 % of individuals showed a prostrate form in their longest trunk with no sigificant differences in this trait between sites.

Of the total of 142 individuals recorded in the 12 sites, 31 % had developed inflorescences and only 12 % had developed fruits. The proportion of plants with flowers differed significantly between sites (χ2 L-R test = 61.815; d.f. = 11; P < 0.0001) independent of plant length (χ2 L-R test = 0.34; d.f. = 1; P = 0.5). In three sites no individuals were observed with inflorescences, in another three sites more than 75 % produced inflorescences and in six sites it was less than 50 % (Figure 1). The proportion of plants with flowers increased significantly with an increase in average annual precipitation (adjusted r2 = 0.27; t ratio = 0.2.27; P = 0.04; n = 12). However, neither altitude (adjusted r2 = 0.07; t ratio = 1.37; P = 0.2; n = 12) or latitude (adjusted r2 = 0.03; t ratio = 1.18; P = 0.2; n = 12) had an effect on the production of inflorescences.
Discussion
This study found a high phenotypic variation in stem, rosette and flowering traits throughout the range of Yucca capensis. Total plant length, leaf length and leaf width fluctuated within the values already recorded for the species, indicating that the variation across the geographic range is still within that reported in the original description (Lenz, 1998). In relation to the sister species, the variation found in total length of Y. capensis is lower that that registered for Y. valida (3-10 m; Turner et al., 1995). However, leaf size presents higher variability in Y. capensis (Y. valida: length: 15-35 cm, width: 1.5-2.5 cm, Turner et al., 1995). Lenz (1998) mentions that Y. valida populations close to la Paz, (24.09 N, 110.20 W) show morphological features which suggest a hybrid origin with Y. capensis.
It is possible that both species have accumulated sufficient differences to be considered different evolutionary entities, as indicated by genetic data (Pellmyr et al., 2008). However, reproductive isolation is probably not complete. Future studies comparing vegetative and reproductive phenotypes of both species, as well as assessments of connectivity and genetic mixing between them, including plants from the populations in question, are necessary to test the hypothesis of hybridization.
The morphometric differences found between the different populations of Yucca capensis suggest the potential presence of genetic variation and/or variation in reponse to different environmental conditions, these factors not being mutually exclusive, as the influence of genotype and environment on phenotypic variation can occur simultaneously (Schlichting y Piggliucci, 1998). In an experiment on Y. valida grown on a single plot of land using clones and seeds of different individuals (i.e. genotypes), Arce-Montoya et al. (2006) found that there was a significant difference in plant growth depending on genotype, indicating that there was genetic variation related to the development of the seedlings.
The response of Yucca capensis to environmental differences in each site can generate the expression of subtle variations in morphological traits of populations. Although no relationship was found between phenotypic variation and altitude or latitude, other factors could be affecting it on a small scale. For example, factors related to the degree of slope, soil exposure and soil depth in each site create different microhabitats. The development of plants in sites exposed to direct solar radiation, and therefore having reduced soil humidity, could determine a phenotypic expression different to that of populations which receive a lower level of direct solar radiation or which inhabit areas with greater soil depth and therefore better humidity retention. Finally, traits such as age of individuals and populations can also generate morphological differences. However, in this study, a plant size effect was not observed on the phenotype of Y. capensis, supporting the hypothesis that genetic or phenotypic plasticity factors probably better explain the observed vegetative and reproductive variation.
The physiological response of individuals of a species to the heterogeneous mosaic of environmental conditions can favor the establishment of phenotypes with different life histories throughout its range (Thompson, 2005). In long-lived species, abiotic factors such as rainfall patterns and temperature have been reported to have a relevant influence on flowering patterns (Bochert et al., 2004; Bustamante and Búrquez, 2008). In natural populations of Yucca capensis, the reported flowering period is between July and October, corresponding to the regional rainy season (Lenz, 1998). In an experimental population of Y. capensis, in which humidity was not a limiting factor due to continuous irrigation, an abundance of flowering was observed during the 15 years of study (Lenz, 1998). The results strongly suggest that presence and levels of rainfall have an influence on flowering patterns in individuals, even when the difference in the amount of precipitation between regions does not seem to be very high (approximately 29 %, Figure 1). In the related species Yucca elata (Engelm.) Engelm. and Hesperoyucca whipplei (Torr.) Baker ex Trel., a strong association has been detected between precipitation and inflorescence, flower and fruit production (Smith and Ludwig, 1976; Udovic, 1981). This supports the hypothesis that levels of rainfall, in conjunction with humidity retention in microhabitats, could explain the variations in flowering patterns within and between the populations observed in this study.
The effect of rainfall on the differential production of inflorescences has important implications for the evolutionary ecology of Yucca capensis. Firstly, the production of flowers represents the first opportunity to produce offspring with genetic variation within populations. Considering that in another Yucca species it has been demonstrated that offspring derived from seed possess advantages in terms of growth compared to the offspring derived from asexual reproduction (Arce-Montoya et al., 2006), the occurrence and regularity of precipitation could play an important role in population dynamics through the contribution and formation of seeds, germination and the establishment of individuals with a different genetic constitution. Secondly, inflorescence production in conjunction with fruit production observed in some populations are clear evidence of the occurrence of the obligated Yucca-moth interaction. In this way, rainfall, by favouring inflorescence production, contributes to the continuation of this interaction in the region. Future molecular studies could test whether yucca populations and their pollinators possess differences at the genetic level in sites with different rainfall patterns.
This study demonstred that there is a large phenotypic variation in vegetative traits and a clear influence of rainfall levels on the production of reproductive structures throughout the range of the endemic species, Yucca capensis. Knowledge of reproductive phenology of this species and the effects of environmental and biotic variables, as well as the influence of sexual and asexual reproduction on the demography of populations will be fundamental to gain an understanding of the phenotypic and genotypic variation of this species. Future studies are needed to determine and compare levels of genetic variation across altitudinal and latitudinal distribution of the species. Connectivity between populations due to the movement of pollen and seed dispersion should also be considered in order to begin to understand the patterns of local adaptation and genetic flow in these populatinos. Finally, comparative evaluations between morphological and genetic variation of Y. capensis and Y. valida throughout their geographic ranges would be of great help towards evaluating the independence of each of these evolutionary entities.
Acknowledgments
We thank Cynthia R. Alamo and Carlo G. González for their invaluable assistance in data processing and fieldwork. We thank two anonymous reviewers for helpful suggestions and comments to improve the quality of this manuscript. We thank R. Hazlewood for the manuscript translation. M.C.A. thanks to CONACYT for the funding project CB-2014-01 238843 and the Rufford Foundation for having provided financial support to develop the study. R.B.B. thanks to CONACYT for the funding project infra-20141 226339 and to the Facultad de Ciencias of UABC for the facilities to conduct this study.
Literature cited
Arce-Montoya M., Rodríguez-Álvarez M., Hernández-González J.A. and Robert M.L. 2006. Micropropagation and field performance of Yucca valida. Plant Cell Reports 25:777-783. [ Links ]
Baker H.G. 1986. Yuccas and yucca moths-A historical commentary. Annals of the Missouri Botanical Garden 73:556-564. [ Links ]
Borchert R., Meyer S.A., Felger R.S. and Porter-Bolland L. 2004. Environmental control of flowering periodicity in Costa Rican and Mexican tropical dry forest. Global Ecology and Biogeography 13:409-425. [ Links ]
Bustamante E. and Búrquez A. 2008. Effects of plant size and weather on the flowering phenology of the organ pipe cactus (Stenocereus thurberi). Annals of Botany 102:1019-1030. [ Links ]
ESRI. 2009. ArcGis (v 9.3). Environmental Systems Research Institute, Redlands. California. [ Links ]
Lambers H. and Poorter H. 1992. Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Advances in Ecological Research 23:188-261. [ Links ]
León de la Luz J.L., Domínguez-Cadena R. and Medel-Narváez A. 2012. Florística de la selva baja caducifolia de la Península de Baja California, México. Botanical Sciences 90:143-162. [ Links ]
León-de la Luz J.L. and Breceda A. 2006. Using endemic plant species to establish critical habitats in the Sierra de La Laguna Biosphere Reserve, Baja California Sur, Mexico. Biodiversity and Conservation 15:1043-1055. [ Links ]
Lenz L.E. 1998. Yucca capensis (Agavaceae, Yuccoideae), a new species from Baja California Sur, Mexico. Cactus and Succulent Journal 70:289-296. [ Links ]
Pellmyr O., Segraves K.A., Althoff D.M., Balcázar-Lara M. and Leebens-Mack J. 2007. The phylogeny of yuccas. Molecular Phylogenetics and Evolution 43:493-501. [ Links ]
Pellmyr O., Balcázar-Lara M., Segraves K.A., Althoff D.M. and Littlefield R.J. 2008. Phylogeny of the pollinating yucca moths, with revision of Mexican species (Tegeticula and Parategeticula; Lepidoptera, Prodoxidae). Zoological Journal of the Linnean Society 152:297-314. [ Links ]
Schlichting C.D. and Piggliucci M. 1998. Phenotypic Evolution: A Reaction Norm Perspective. Sinauer Associates, Sunderland. [ Links ]
Smith S.D. and Ludwig J.A. 1976. Reproductive and vegetative growth patterns in Yucca elata Engelm. (Liliaceae). The Southwestern Naturalist 21:177-184. [ Links ]
Sosa V. and De-Nova J.A. 2012. Endemic angiosperm lineages in Mexico: Hotspots for conservation. Acta Botanica Mexicana 100:293-315. [ Links ]
Thompson N.J. 2005. The Geographic Mosaic of Coevolution. The University of Chicago Press, Chicago. [ Links ]
Turner R.M., Bowers J.E. and Burgess T.L. 1995. Sonoran Desert Plants: an Ecological Atlas. University of Arizona Press, Tucson. [ Links ]
Udovic D. 1981. Determinants of fruit set in Yucca whipplei: reproductive expenditure vs. pollinator availability. Oecologia, 48:389-399. [ Links ]
Whitlock M.C. and Schluter D. 2009. The Analysis of Biological Data. Roberts and Company Publishers, Greenwood Village. [ Links ]














