Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Botanical Sciences
versão On-line ISSN 2007-4476versão impressa ISSN 2007-4298
Bot. sci vol.92 no.1 México Mar. 2014
Ecología
Soil seed bank, seed removal, and germination in a seasonally dry tropical forest in Veracruz, Mexico
Banco de semillas del suelo, remoción y germinación de semillas en una selva baja caducifolia en Veracruz, México
Claudia Alvarez-Aquino1, Laura Barradas-Sánchez1, Oscar Ponce-González1 and Guadalupe Williams-Linera2,3
1 Instituto de Investigaciones Forestales; Universidad Veracruzana, Xalapa, Veracruz, Mexico
2 Instituto de Ecología, A.C. Xalapa, Veracruz, Mexico
3 Corresponding author: guadalupe.williams@inecol.mx
Received: February 11th, 2013
Accepted: March 22nd, 2013
Abstract
The soil seed bank has a limited role in the seasonal dry tropical forest regeneration process, but seed removal and germination can also be limiting factors during the early forest recovery. In central Veracruz, Mexico, the soil seed bank was determined en five fallows and two forests. Seed bank decreased from fallow to forest (1,303 to 101 seeds m-2); herbs and grasses predominated, thus the similarity between species composition of seed bank and vegetation was low. Seed removal and germination were evaluated for Acacia cochliacantha, Caesalpinia cacalaco, lpomoea wolcottiana, and Senna alomaría, in contrasting habitats represented by pasture, fallow and forest. Seed removal was determined under treatments of total access, rodent exclosure, and insect exclosure. Caesalpinia (largest seeds) showed the lowest seed removal (5%), whereas Senna (63%) and lpomoea (29%) showed the highest. Rodent exclosure reduced seed removal for lpomoea (medium-sized seeds); and insect exclosure reduced removal for Senna and Acacia (small seeds). With the exception of Senna (18% germination), the scarified seeds displayed the highest germination percentage (53-99%). For all species, germination was higher in forest than in open habitats, only Senna seeds presented the lowest germination percentage in the forest habitat. Results suggested that in the dry forest of Veracruz, current seed removal may not limit forest regeneration. We suggest seed scarification of some species for use in restoration activities.
Key words: dry forest, forest recovery, native trees, seed predation, seed scarification, secondary succession.
Resumen
El banco de semillas del suelo tiene un papel limitado en la recuperación de la selva baja caducifolia, pero la remoción y germinación de semillas pueden también ser factores limitantes al inicio de la sucesión. En el centro de Veracruz, México, el banco de semillas del suelo se determinó en cinco acahuales y dos selvas. El banco de semillas disminuyó de acahual a selva (1,303 a 101 semillas m-2). Predominaron hierbas y pastos, por lo que la similitud entre la composición de especies del banco y la vegetación fue baja. La remoción y germinación de semillas se evaluó en Acacia cochliacantha, Caesalpinia cacalaco, lpomoea wolcottiana y Senna atomaria en hábitats contrastantes representados por potrero y selva. La remoción de semillas se determinó bajo tratamientos de acceso total, exclusión de roedores y de insectos. Caesalpinia (semilla más grande) presentó la menor remoción (5%), mientras que Senna (63%) e lpomoea (29%) presentaron la más elevada. La exclusión de roedores redujo la remoción para Ipomoea (semillas de tamaño mediano), y la exclusión de insectos redujo la remoción de Senna y Acacia (semillas pequeñas). Con la excepción de Senna (18% germinación), las semillas escarificadas presentaron el mayor porcentaje de germinación (53-99%). Para todas las especies, la germinación fue mayor en selva que en hábitats abiertos, únicamente Senna presentó un porcentaje de germinación más bajo en selva. La remoción actual de semillas puede no limitar la regeneración. Se recomienda la escarificación de las semillas de algunas especies para actividades de restauración.
Palabras clave: árboles nativos, depredación de semillas, escarificación de semillas, recuperación de selva, selva seca, sucesión secundaria.
Soil seed banks, as well as seed removal and germination, have been studied in relation to the recovery of the seasonally dry tropical forest in abandoned fields (Rico-Gray and García-Franco, 1992; Skoglund, 1992; Miller, 1999; Wijdeven and Kuzee, 2000; Dalling, 2002; Lemenih and Teketay, 2006; Reubens et al., 2007; Maza-Villalobos et al., 2011; Meave et al., 2012). In dry forests, another important source of forest regeneration is root or stem sprouting (Vieira and Scariot, 2006); however, when propagules are available, forest recovery in open areas may occur through secondary succession (Garwood, 1989; Dalling and Denslow, 1998; Holl, 1999; Holl et al., 2000; Williams-Linera et al., 2011).
The presence of propagules can be the result of seed storage in soil banks, seed rain, and the proximity to the propagule source-forest patches, isolated trees, or secondary vegetation (Holl, 1999; Zahawi and Augspurger, 1999; Wijdeven and Kuzee, 2000; Vieira and Scariot, 2006). Lack of dispersal and competition with seedlings and pastures may limit natural regeneration (Holl et al., 2000; Maza-Villalobos et al., 2011; Reid and Armesto, 2011). In abandoned areas, seed banks can trigger secondary succession; however, successful seedling establishment is influenced by other selective pressures that affect regeneration patterns, such as animal-plant interactions (e.g., herbivory, seed removal), seed germination, and favorable microenvironments (Skoglund, 1992; Holl et al., 2000; Fenner and Thompson, 2005; Aerts et al., 2006; Wassie et al., 2009).
Seed predation may critically reduce seedling recruitment; therefore, postdispersal seed removal has been identified as a crucial phase in the regeneration and composition of tropical dry forest communities (Wijdeven and Kuzee, 2000; Jones et al., 2003; Briones-Salas et al., 2006). A general consensus is that low seed availability in open areas and seed mortality during dispersal are two of the major constraints to natural regeneration (Hammond, 1995; Vieira and Scariot, 2006). Seed size is also an important factor that affects the chances of being eaten or removed. Rodents prefer larger seeds, while small ones are more likely to become buried, which may cause ants and beetles to preferentially select them (Forget and Milleron, 1991; Dalling, 2002; Fenner and Thompson, 2005; Briones-Salas et al., 2006; Farwig et al., 2008).
Seed germination studies have found more variability between tree species than between habitats (Baskin et al., 1998; Holl, 2002). A higher germination rate in sites with vegetation cover than in open areas has been found; there, high humidity and low evapotranspiration may provide more favorable microclimatic conditions (Holl, 1999; Ceccon et al., 2006; Wassie et al., 2009). Mechanical or chemical scarification or other damage to seed coatings allows water absorption and promotes germination (Camargo-Ricalde and Grether, 1998; Ortega et al., 2001; Dalling et al., 2011).
In central Veracruz, the seasonally dry tropical forest (SDTF) has been reduced to 7% of its original size, even though one-third of this area is secondary vegetation; previous work has indicated that the fallow period is short, usually no longer than 7-10 years, making old secondary succes-sional sites unavailable (Williams-Linera et al., 2011). The working hypothesis were: (1) the size and composition of soil seed banks in successional sites and dry forest habitats do not mirror the aboveground vegetation, (2) the seed removal patterns of selected tree species is higher in open habitats, and (3) the germination potential of selected tree species in contrasting habitats (active pasture, successional sites and forest) will be lower in open habitats.
Methods
Study area. The study area is located in the SDTF region of central Veracruz, Mexico (19° 17' N, 96° 26' W; 19° 10' N, 96° 32' W; altitude: 97-230 m). In forests fragments, some dominant tree species are Bursera fagaraoides, B. simaruba, Calyptranthes schiedeana, Heliocarpus donnellsmithii, lpomoea wolcottiana, Luehea candida, and Tabebuia chrysantha, whereas in the early successional sites, dominant species are Acacia cochliacantha, A. cornigera, and Guazu-ma ulmifolia. Mean annual precipitation is 957 mm (range: 502-1,467 mm), with a dry season from October to May. Mean minimum and maximum temperatures are 20 and 31 °C, respectively. The dominant soil units are Cambisol and Vertisol with exposed rock. In this area, we selected eight study sites corresponding to different habitats: one active pasture (P1), five fallows with different years of abandonment (S1-S5), and two forest fragments (F1-F2; Table 1). A detailed description of each site can be found in Williams-Linera and Lorea (2009) and Williams-Linera et al. (2011).
Soil seed bank. The soil was collected from five fallow sites and two forest fragments in late May 2008, just before the onset of the rainy season that triggers seed germination (Table 1). At each study site, ten soil samples (30 cm x 30 cm and 5 cm depth) were collected on plots previously established for vegetation studies (Williams-Linera and Lorea, 2009; Williams-Linera et al., 2011). The soil samples were transported to a greenhouse located on the campus of the University of Veracruz in Xalapa. They were placed in 70 trays (53 x 26 cm) in layers of approximately 5 cm depth. Four control trays with autoclaved sterilized soil were located randomly in the greenhouse. Trays were watered every day during the seven-month period. Emerging seedlings were identified, counted and removed from the trays to reduce shade. Unidentified seedlings were transplanted to pots and grown until identification became possible; seedlings were identified and deposited in the University of Veracruz XALUV herbarium. Although dormant seeds may not show using this technique, germination may provide a more reliable method than directly extracting and counting the seeds from the soil (Gross, 1990).
Plant species. Four native tree species were selected to test germination and seed removal. They were abundant and represented different seed dispersal modes: Acacia cochlia-cantha (animal-dispersed), Ipomoea wolcottiana (wind-dispersed), and Caesalpinia cacalaco and Senna atomaria (self-dispersed). Acacia cochliacantha and S. atomaria can be considered as early successional or pioneer trees and the other species as late succesional or forest species (Williams-Linera et al., 2011). Hereafter, they will be referred to by genus only. Seeds were collected between May and August 2007, when fruit production peaks (GWL, unpubl. data).
Five to ten mature trees of each species were selected, and at least 50 fruits were collected per tree and stored in paper bags. Seeds were separated from fruit; only seeds that had a good appearance (fully grown and without evidence of rot or insect damage) were chosen and subsequently selected using the flotation in water method.
Since the probability of successful seedling establishment in the face of environmental hazards increases with seed mass, the seed volume was calculated from linear dimensions, assuming an ellipsoidal shape and using the equation proposed by Dias and Ganhao (2012). Ten seeds of each species were randomly chosen and measured to the nearest mm. Seed mass varied by 5-6 orders of magnitude among species. Caesalpinia seeds had the largest volume (V = 243.8 mm3, se = 17.3), followed by lpomoea (V = 109.8 mm3, se = 5.4); seeds of Senna (V = 21.1 mm3, se = 1.2) and Acacia (V = 17.3 mm3, se = 2.5) were smaller and statistically similar to each other (Kruskal-Wallis test, χ2 = 34.2, P < 0.0001).
Seed removal. Seed removal was studied during the rainy season using three treatments: total access to animals, and the exclusion of rodents or exclusion of insects. To exclude insects, we used 20 x 20 x 10 cm boxes made of wire mesh with 0.5 cm2 openings, and open tops and walls coated with Vaseline to keep insects from entering. To exclude rodents, we used 20 x 20 x 10 cm boxes made of wire mesh with 1 cm2 openings and closed tops. At site P1, twelve trees were chosen in open areas. Boxes were placed at ground level under the crown of each tree at least 0.50 m from the trunk. For each species, 25 seeds were placed in one Petri dish per box; three treatments with four replications were located around each tree. A total of 144 boxes (per species, 12 open, 12 inaccessible to insects, and 12 inaccessible to rodents) were checked daily during the first week and every other day for the following three weeks.
Seed germination. Seed germination experiments were conducted in laboratory and field conditions using two treatments consisting of mechanical scarification and one control. Scarification consisted of a small cut in the seed coat made with a knife, care being taken not to damage the embryo. Control and scarified seeds were immersed in distilled water for a period of 24 hours. Subsequently, sets of 50 seeds were placed in four Petri dishes for each treatment; cotton was used as substrate and kept wet with distilled water to a saturation point during the whole experiment. In the lab, germination was evaluated in a germination chamber (Lab-Line Instruments INC.) with temperature varying from 25 to 30 °C, and 12-hour photoperiod. Germination in the field was conducted in forest, fallow, and pasture habitats during the rainy season. In each, we established four 1 x 1 m plots, one for each species. Eight Petri dishes (four with control seeds and four with scarified seeds) were placed on each plot. The 96 Petri dishes were buried at 3 cm with removal of litterfall; they were protected with wire mesh boxes 20 x 20 x 10 cm placed at ground level. We counted germinated seeds daily for one week and then every other day.
Statistical analyses. Differences in soil seed bank density and richness were analyzed using generalized linear models, since data were counts a Poisson distribution and logarithmic link function were used to test differences in total density, richness, and life forms. Shannon diversity indices were estimated as H' = - 2p. ln p.. The Chao-Jaccard Index was calculated to analyze similarity between seed bank species abundance using Estimates version 7.5 software (Colwell, 2005). This index has a value of 1 for identical species composition and 0 when assemblages are completely dissimilar. Absence-presence of species between seed banks and woody vegetation were compared, but since they were predominantly different, the index was not calculated.
For the seed removal experiment, all seeds were observed during a month; survivorship curves were analyzed as censored data with the product-limit (Kaplan-Meier) survival analysis. This is a nonparametric test for comparing the distribution of life-spans of groups; Wilcoxon statistics was used to test homogeneity between groups. Statistical analyses were performed using JMP version 6.0 software.
Germination data percentages were arcsine transformed, and two-way ANOVAs were used to test for differences between treatments (scarification and control) and habitats (forest, fallow, and pasture) per species. When significant differences were detected, means were compared using a Tukey-HSD test.
Results
Seed bank. In total, 3,946 seedlings were recorded in the soil seed bank of the study sites, corresponding to 25 families represented by 51 genera, 83 species, and three morphospecies, although graminoid species (grasses and sedges) were not identified (Appendix 1). The most abundant families were Cyperaceae-Poaceae (47.7%), Acanthaceae (15.2%), Euphorbiaceae (9.9%), Malvaceae (6.9%), Asteraceae (6.6%), and Fabaceae (1.8%). Life forms were unequally represented, with very few trees and abundant herbs (including sedges and grasses; Figure 1). Seed density ranged from 101 to 1,303 seeds m-2. Overall, germinated seeds significantly decreased from the most disturbed site (S1) to the forest (F1 and F2) (Figure 1A). Richness varied from 13 to 36 species (Figure 1B), and the Shannon diversity index was between 0.93 and 2.41 (Figure 1C). When soil seed bank composition was compared using the Chao-Jaccard Index, we found that fallows S2, S3, and S4 were highly similar. Furthermore, the soil seed bank of the two forest sites was similar between themselves and S4 too (Table 2). However, seed bank in fallow S1 differed from all others.
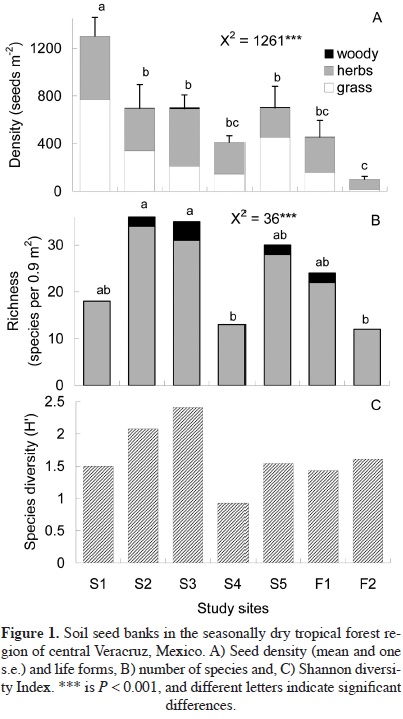

The species composition found in soil seed banks and woody vegetation was dissimilar (Chao Jaccard Index = 0). Only Acacia cochliacantha, Bursera cinerea, and Caesalpinia cacalaco were found in both the seed bank and standing vegetation of the same site. However, seeds from species not present as adult plants such as Ipomoea wolcottiana, Piscidia piscipula, and Senna atomaria were found in seed banks (Appendix 1).
Seed removal. Seed removal was significantly different among species (χ2 = 1493, df = 3, P < 0.0001) and treatments (χ2 = 409, df = 2, P < 0.0001). Overall, Senna and lpomoea had the highest percentage of seed removal; as could be expected, for all species the highest percentage of seed removal was recorded when seeds were offered with complete access to animals (Figure 2). The species with the largest seeds (Caesalpinia) showed the lowest seed removal rate (Figure 2A). The treatment of rodent exclosure reduced seed removal for lpomoea, which has medium-sized seeds (Figure 2B); the insect exclosure treatment reduced seed removal for species with small seed size, such as Senna (Figure 2C) and Acacia (Figure 2D).

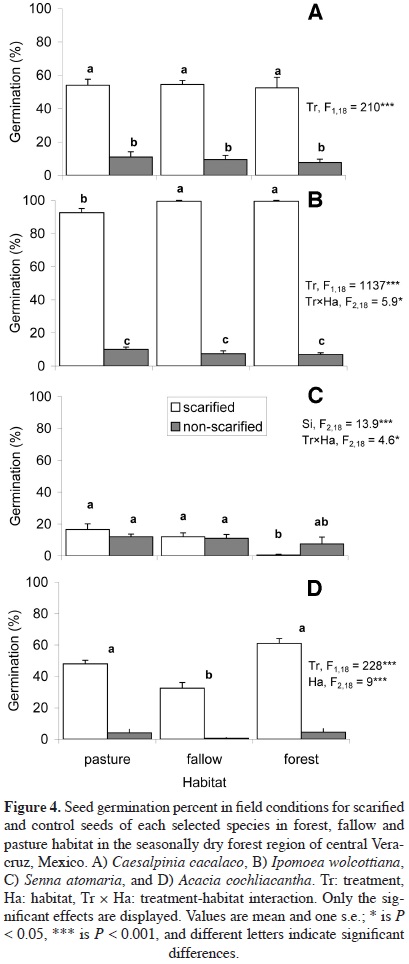
Seed germination. Under laboratory conditions, seed germination was significantly higher for seeds with mechanical scarification treatment than for untreated seeds, and was significantly different among species (Figure 3). For scarified seeds, the highest percentages were recorded for lpomoea (99.0%) and Caesalpinia (98.5%) seeds, followed by Acacia (81.0%) and Senna (18.5%). In field conditions, scarified seeds also had a significantly higher germination percent for all species (47.2-97.2%) except Senna (9.7%; Figure 4). Species showed differences in germination percentage. Caesalpinia did not display significant differences between habitats (Figure 4A), while Ipomoea seed germination was higher in fallow and forest than in pasture (Figure 4B). Germination percentage for Acacia seeds (Figure 4D) was higher in forest and pasture than in the fallow habitat, whereas germination in the forest was the lowest for Senna seeds (Figure 4C).
Discussion
Soil seed bank density in early successional and forest habitats was within the range found for tropical dry forests, with a trend toward more germinated seeds at more disturbed sites (70-855 seed m-2, Rico-Gray and García-Franco,1992; 700-1,000 seeds m-2, Miller, 1999; 466-1,257 seed m-2, Uasuf et al., 2009; 806-3,150 seed m2, Maza-Villalobos et al., 2011). In our study, land-use history does not have a clear effect on seed bank characteristics, however, a site used for agriculture for a long time (S1, Table 1) had the highest seed density and lower richness than the abandoned sites planted with exotic grasses and the forest sites. In Ethiopia, changes in soil seed density did not show any trend with cultivation time (Lemenih and Teketay, 2006), but in Oaxaca, Mexico, Meave et al. (2012) found that seed bank density tended to increase with the time of use of the agricultural fields. Whereas, along a chronosequence of abandoned pastures and forest sites in Chamela, Mexico, Maza-Villalobos et al. (2011) reported a reduction in the seed density.
Previous studies have shown that the species composition of soil seed banks in SDTF and successional sites are dominated by grasses and herbs, with scarce native forest species (Garwood, 1989; Rico-Gray and García-Franco, 1992; Miller, 1999; Tekle and Bekele, 2000; Lemenih and Teketay, 2006; Reubens et al., 2007; Salazar et al., 2011; Meave et al., 2012). Here, a few woody species were found in the soil bank, mostly at the successional site (S3) with high species richness both in the seed bank and vegetation; however, the soil seed bank on the forest habitats contained just two seeds from woody species. Similarity between the composition of seed banks and woody vegetation was near zero. Soil was collected before the rainy season to sample the transient plus persistent bank (Fenner and Thompson, 2005); thus, scarceness of woody species may indicate that SDTF species did not form a persistent seed bank and germinate after dispersal during the rainy season (Salazar et al., 2011). At early successional sites, the limited representation of woody species in seed banks may be due to their distance from propagule sources (Garwood, 1989; Wijdeven and Kuzee, 2000; Lemenih and Teketay, 2006; Salazar et al., 2011), absence of seed dispersers such as birds (Jiménez and Armesto, 1992; Rico-Gray and García-Franco, 1992), high predation rates (Hammond, 1995; Jones et al., 2003; Briones-Salas et al., 2006), or the use of fire for land clearing (Miller, 1999).
In our study, some tree species were found in both the seed bank and vegetation, while others species were identified in the soil but not in standing vegetation. Those seeds may have arrived due to seed rain, since nearby forest patches, isolated trees that provide shade for livestock, and living fences could act as propagule sources (Holl, 1999; Ceccon et al., 2006). Seed banks have been considered a limited tool in regenerating dry forest vegetation because an almost total absence of seeds from woody species (Lemenih and Teketay, 2006; Reubens et al., 2007; Uasuf et al., 2009; Reid and Armesto, 2011; Salazar et al., 2011). However, in Nicaragua, Uasuf et al. (2009) found a 20% similarity between seed bank and standing vegetation; in Tanzania, Lyaruu et al. (2000) reported several species in common, and in Chamela, Mexico, Maza-Villalobos et al. (2011) found that during succession the soil seed banks change and, herb and grass species may be replaced in abundance and diversity by woody species.
Seed removal may produce considerable seed losses (Wijdeven and Kuzee, 2000; Briones-Salas et al., 2006).
In this study, the highest seed removal rate occurred in the total access treatment, as has been observed in other SDTF studies (e.g., Hammond, 1995; Briones-Salas et al., 2006). Species removal agents may be small mammals such as rodents or invertebrates such as ants. Here, insects seem to be the most important seed removers. In Chiapas, Hammond (1995) found that seed removal by vertebrates is greater in early successional sites after shifting agriculture than in mature forest or at sites abandoned for more than 30 years. In Costa Rica, Jones et al. (2003) reported more removal in abandoned pasture adjacent to forest fragments than far from the edge; and that interspecific differences in seed removal rates were consistent with the hypothesis that in pasture, larger seeds are removed less than smaller seeds. In our study, the largest seeds are relatively small compared to the seeds of species that occur in secondary forest (Khurana and Singh, 2001; Fenner and Thompson, 2005). Nevertheless, Caesalpinia, the species with the largest seeds, was removed, or at least, despite the fact that it has been found as one of the most removed seed by rodents in Chamela (Briones-Salas et al., 2006) and, that rodents are abundant in our study area and are represented by at least eight species (Gómez-Alanis, 2010). lpomoea (medium-size seed) and Senna (small-size seed) had the highest percentage of seed removal. In the case of Ipomoea seeds, a lower removal with rodent exclosure suggests that rodents were the main predator.
In addition, direct field observations indicated that ants also remove these seeds, as substantial amounts of seeds were found around ant nests. Ipomoea presented a vast seed production that can be directly related to the observed high seed removal (CAA, pers. obs). Several authors have found this denso-dependent mechanism of higher removal rates when seed density is high (Hammond, 1995; Fenner and Thompson, 2005; Briones-Salas et al., 2006).
In this study, seed removal was relatively low in comparison with values reported for other SDTF sites (98%, Hammond, 1995; 42%, Wijdeven and Kuzee, 2000); thus, current removal rates may not be a limiting factor for regional forest regeneration. Still, before seed germination and seedling establishment take place other barriers such as seed scarification and microhabitat became apparent. Mechanical seed scarification resulted in substantially higher germination percentage, both in lab and field conditions, except for Senna seeds. Our results suggest that the other species (Acacia, Caesalpinia, and Ipomoea) need to be mechanically scariied to absorb water to enhance germination; otherwise, germination percentage is low and spread out over time. Senna as a pioneer tree, displayed lower germination in the shaded forest habitat, but also in the light-controlled germination chamber, thus Senna seeds may have a non-physical dormancy and variation in seed dormancy among populations (Lacerda et al., 2004).
Caesalpinia displayed similar germination percentage in different habitats, whereas Ipomoea and Acacia had higher germination in forest habitat. In SDTF, germination and early seedling establishment mainly depend on water availability; therefore, lower germination in pasture and fallow are probably due to water stress (Lieberman and Li, 1992; Fenner and Thompson, 2005; Ceccon et al., 2006; Wassie et al., 2009). Scarification is required because the hard and impermeable seed coat prevents the passage of water to the embryo (Cervantes et al., 1996; Camargo-Ricalde and Grether, 1998; Fenner and Thompson, 2005; Wassie et al., 2009; Maza-Villalobos et al., 2011). Seed dormancy prevails in SDTF species, but increased germination after mechanical scariication has been reported for several legume tree seeds (Acacia cochliacantha, Cervantes et al., 1996; Mimosa tenuiflora, Camargo-Ricalde and Grether, 1998; Caesalpiniaparaguariensis, Ortega et al., 2001). Under natural conditions, scariication may depend on the effect of avian gut-passage (Reid and Armesto, 2011), but other authors have suggested that in a dry environment, temperature fluctuations on the soil surface can soften the seed coat and allow germination (Moreno-Casasola et al., 1994; Ortega et al., 2001; Fenner and Thompson, 2005).
Conclusions
This study suggests that the soil seed bank of disturbed SDTF habitats is very unlikely to contribute to forest recovery since there is no resemblance between the species composition of seed bank and vegetation. The effect of seed removal may not be a limiting factor for recovery in open areas: most seeds of the selected species suffered low removal rates. Furthermore, several seed species showed physical dormancy that broke after scarification. For restoration activities, seeds need to be mechanically scarified in order to achieve a high germination rate. In the study area, the presence of woody vegetation and the proximity to forest fragments may provide early support for forest recovery.
Acknowledgments
We are grateful to Javier Tolome, Marichu Peralta, and Alfonso Suárez Islas for their valuable help and friendship. We thank Enrique Jurado and an anonymous referee for their helpful suggestions that improved this manuscript. We are grateful to the landowners who permitted fieldwork on their properties: Miguel Morales in Paso de Ovejas and Don Aurelio Molina and son in Xocotitla. This research was funded by the European Community under INCO Project ReFor-Lan CT2006-032132.
Literature cited
Aerts R., Maes W., November E., Negussie A., Henry M. and Muys B. 2006. Restoring dry Afromontane forest using bird and nurse plant effects: direct sowing of Orea europaea ssp. cuspidata seeds. Forest Ecology and Management 230:23-31. [ Links ]
Baskin J.M., Nan X. and Baskin C.C. 1998. A comparative study of seed dormancy and germination in an annual and a perennial species of Senna (Fabaceae). Seed Science Research 8:501-512. [ Links ]
Briones-Salas M., Sánchez-Cordero V. and Sánchez-Rojas G. 2006. Multi-species fruit and seed removal in a tropical deciduous forest in Mexico. Canadian Journal of Botany 84:433-442. [ Links ]
Camargo-Ricalde S.L. and Grether R. 1998. Germinación, dispersión y establecimiento de plántulas de Mimosa tenuiflora (Legu-minosae) en México. Revista de Biología Tropical 46:543-554. [ Links ]
Ceccon E., Huante P. and Rincón E. 2006. Abiotic factors influencing tropical dry forests regeneration. Brazilian Archives of Biology and Technology 49:305-312. [ Links ]
Cervantes V., Carabias J. and Vázquez-Yañez C. 1996. Seed germination of woody legumes from deciduous tropical forest of southern Mexico. Forest Ecology and Management 82:171-184. [ Links ]
Colwell R.K. 2006. EstimateS: Statistical estimation of species richness and shared species from samples. Version 7.5. [ Links ]
Dalling J.W. 2002. Ecología de semillas. In: Guariguata M.R. and Kattán G. Eds. Ecología y Conservación de Bosques Neotropicales, pp. 345-375, Libro Universitario Regional, Cártago. [ Links ]
Dalling J.W. and Denslow J.S. 1998. Soil seed bank composition along a forest chronosequence in seasonally moist tropical forest, Panama. Journal of Vegetation Science 9:669-678. [ Links ]
Dalling J.W., Davis A.S., Schutte B.J. and Arnold A.E. 2011. Seed survival in soil: interacting effects of predation, dormancy and the soil microbial community. Journal of Ecology 99:89-95. [ Links ]
Dias L.S. and Ganhao E. 2012. Extending the range for accurate estimation of seed volume from incomplete linear dimension data. Seed Science and Technology 40:129-133. [ Links ]
Farwig N., Bleher B., von der Gónna S. and Bóhning-Gaese K. 2008. Does forest fragmentation and selective logging affect seed predators and seed predation rates of Prunus africana (Ro-saceae)? Biotropica 40:218-224. [ Links ]
Fenner M. and Thompson K. 2005. The Ecology of Seeds. Cambridge University Press, Cambridge. [ Links ]
Forget P.M. and Milleron T. 1991. Evidence for secondary seed dispersal by rodents in Panama. Oecologia 87:596-599. [ Links ]
Garwood N.C. 1989. Tropical soil seed Banks: a review. In: Allessio L.M., Thomas P.V. and Simpson R.L. Eds. Ecology of Soil Seed Banks, pp. 149-209, Academic Press, San Diego. [ Links ]
Gómez-Alanis, C. 2010. Análisis multicriterio para la determinación de áreas prioritarias para la conservación de elementos bióticos y culturales en el bosque seco del centro de Veracruz, México. Master Thesis. Instituto de Ecología, A.C., Xalapa. 135 pp. [ Links ]
Gross K.L. 1990. A comparison of methods for estimating seed numbers in the soil. Journal of Ecology 78:1079-1093. [ Links ]
Hammond D.S. 1995. Post-dispersal seed and seedling mortality of tropical dry forest trees after shifting agriculture, Chiapas, Mexico. Journal of Tropical Ecology 11:295-313. [ Links ]
Holl K.D. 1999. Factors limiting rain forest regeneration in abandoned pasture: seed rain, seed germination, microclimate, and soil. Biotropica 31:229-242. [ Links ]
Holl K.D. 2002. Effect of shrubs on tree seedling establishment in an abandoned tropical pasture. Journal of Ecology 90:179-187. [ Links ]
Holl K.D., Loik M.E, Lin E.H.V. and Samuels I.A. 2000. Tropical montane forest restoration in Costa Rica: overcoming barriers to dispersal and establishment. Restoration Ecology 8:339-349. [ Links ]
Jiménez H.E. and Armesto J. 1992. Importance of the soil seed bank of disturbed sites in Chilean matorral in early secondary succession. Journal of Vegetation Science 3:579-586. [ Links ]
Jones F.A., Peterson C.J. and Haines B.L. 2003. Seed predation in Neotropical premontane pastures: site, distance, and species effects. Biotropica 35:219-225. [ Links ]
Khurana E. and Singh J.S. 2001. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: a review. Environmental Conservation 28:39-52. [ Links ]
Lacerda D.R., Filho J.P.L., Goulart M.F., Ribeiro R.A. and Lovato M.B. 2004. Seed-dormancy variation in natural populations of two tropical leguminous tree species: Senna multijuga (Caesal-pinoideae) and Plathymenia reticulata (Mimosoideae). Seed Science Research 14:127-135. [ Links ]
Lemenih M. and Teketay D. 2006. Changes in soil seed bank composition and density following deforestation and subsequent cultivation of a tropical dry Afromontane forest in Ethiopia. Tropical Ecology 47:1-12. [ Links ]
Lieberman D. and Li M. 1992. Seedling recruitment patterns in a tropical dry forest in Ghana. Journal of Vegetation Science 3:375-382. [ Links ]
Lyaruu H.V., Eliapenda S. and Backéus I. 2000. Floristic, structural and seed bank diversity of a dry Afromontane forest at Mafai, central Tanzania. Biodiversity and Conservation 9:241-263. [ Links ]
Maza-Villalobos S., Lemus-Herrera C. and Martínez-Ramos M. 2011. Successional trends in soil seed banks of abandoned pastures of a Neotropical dry region. Journal of Tropical Ecology 27:35-49. [ Links ]
Meave J.A., Flores-Rodríguez C., Pérez-García E.A. and Romero-Romero M.A. 2012. Edaphic and seasonal heterogeneity of seed banks in agricultural fields of a tropical dry forest region in Southern Mexico. Botanical Sciences 90:313-329. [ Links ]
Miller PM. 1999. Effects of deforestation on seed banks in a tropical deciduous forest of western Mexico. Journal of Tropical Ecology 15:179-188. [ Links ]
Moreno-Casasola P., Grime J.P. and Martínez M.L. 1994. A comparative study of the effects of fluctuations in temperature and moisture supply on hard coat dormancy in seeds of coastal tropical legumes in Mexico. Journal of Tropical Ecology 10:67-86. [ Links ]
Ortega B.P., De Viana M.L., Larenas G. and Saravia M. 2001. Germinación de semillas de Caesalpinia paraguariensis (Fabaceae): agentes escarificadores y efecto del ganado. Revista de Biología Tropical 49:301-304. [ Links ]
Reid S. and Armesto J.J. 2011. Avian gut-passage effects on seed germination of shrubland species in Mediterranean central Chile. Plant Ecology 212:1-10. [ Links ]
Reubens B., Heyn M., Gebrehiwot K., Hermy M. and Muys. 2007. Persistent soil seed banks for natural rehabilitation of dry tropical forests in northern Ethiopia. Tropicultura 25:204-214. [ Links ]
Rico-Gray V. and García-Franco J.G. 1992. Vegetation and soil seed bank of successional stages in tropical lowland deciduous forest. Journal of Vegetation Science 3:617-624. [ Links ]
Salazar A., Goldstein G., Franco A.C. and Miralles-Wilhelm F. 2011. Timing of seed dispersal and dormancy, rather than persistent soil seed-banks, control seedling recruitment of woody plants in Neotropical savannas. Seed Science Research 21:103-116. [ Links ]
Skoglund J. 1992. The role of seed banks in vegetation dynamics and restoration of dry tropical ecosystems. Journal of Vegetation Science 3:357-360. [ Links ]
Tekle K. and Bekele T. 2000. The role of soil seed banks in the rehabilitation of degraded hillslopes in southern Wello, Ethiopia. Biotropica 32:23-32. [ Links ]
Uasuf A., Tigabu M. and Odén P.C. 2009. Soil seed banks and regeneration of Neotropical dry deciduous and gallery forests in Nicaragua. Bois et Forêts des Tropiques 299:49-62. [ Links ]
Vieira D.L.M. and Scariot A. 2006. Principles of natural regeneration of topical dry forests for restoration. Restoration Ecology 14:11-20. [ Links ]
Wassie A., Sterck F.J., Teketay D. and Bongers F. 2009. Tree regeneration in Church forests of Ethiopia: effects of microsites and management. Biotropica 41:110-119. [ Links ]
Wijdeven S.M.J. and Kuzee M.E. 2000. Seed availability as a limiting factor in forest recovery processes in Costa Rica. Restoration Ecology 8:414-424. [ Links ]
Williams-Linera G. and Lorea F. 2009. Tree species diversity driven by environmental and anthropogenic factors in tropical dry forest fragments of central Veracruz, Mexico. Biodiversity and Conservation 18:3269-3293. [ Links ]
Williams-Linera G., Alvarez-Aquino C., Hernández-Ascención E. and Toledo M. 2011. Early successional sites and the recovery of vegetation structure and tree species of the tropical dry forest in Veracruz, Mexico. New Forests 42:131-148. [ Links ]
Zahawi R.A. and Augspurger C.K. 1999. Early plant succession in abandoned pastures in Ecuador. Biotropica 31:540-552. [ Links ]














