Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Botanical Sciences
versión On-line ISSN 2007-4476versión impresa ISSN 2007-4298
Bot. sci vol.91 no.3 México sep. 2013
Fitoquímica
Variability of the Foliar Phenol Profiles of the Agave victoriae-reginae Complex (Agavaceae)
Variabilidad de los Perfiles Fenólicos Foliares del Complejo Agave victoriae-reginae (Agavaceae)
Norma Almaraz-Abarca1,3, María del Socorro González-Elizondo1, María da Graça Campos2, Zeila Eréndira Ávila-Sevilla1, Eli Amanda Delgado-Alvarado1, José Antonio Ávila-Reyes1
1 Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional Unidad Durango, Instituto Politécnico Nacional, Durango, Durango, México
2 Center of Pharmaceutical Studies, Laboratory of Pharmacognosy and Phytotherapy Faculty of Pharmacy, University of Coimbra, Pólo III-Health Sciences, Coimbra, Portugal
3 Correspondence author: e-mail: nalmaraz@ipn.mx.
Received: September 12th, 2012
Accepted: November 16th, 2012
Abstract
The foliar phenol profiles of eight populations of the Agave victoriae-reginae complex were determined by high performance liquid chromatography with diode array detection in order to assess the variability of those compounds across the natural distribution of the complex, and to determine their taxonomical significance. With comparative aims Agave lechuguilla and A. striata were analyzed in the same manner. A total of 81 phenolic compounds were detected, comprising 18 phenolic acids, 51 flavonoids (26 flavonols, 19 dihydrovonoids, and six flavones), and 12 non identified phenols. Each population of the A. victoriae-reginae complex showed a different foliar phenol composition, and A. pintilla, the most recently described species of the group, could be distinguished by four compounds not shared with any other analyzed population. Phenolic acids were detected in all taxa. However, a higher proportion of those compounds was found in the A. victoriae-reginae complex, while a predominance of flavonols (quercetin-3-O-glycosides) was found in A. striata, and a codominance of dihydroflavonoids and flavonols in A. lechuguilla, allowing to distinguish species-specific profiles. Differences in the contents of some phenolic compounds among those three species and within the A. victoriae-reginae complex were also found. Our results sustain the proposal of considering A. victoriae-reginae as a species complex and the separation of A. pintilla as an independent species. The results also reveal the foliar phenol patterns as valuable chemical markers in Agave.
Key words: Agave lechuguilla, Agave striata, Agave victoriae-reginae, chemical variability, phenol profiles.
Resumen
Se determinaron los perfiles fenólicos foliares de ocho poblaciones del complejo Agave victoriae-reginae por medio de cromatografía líquida de alta resolución con detector de arreglo de diodos para evaluar la variabilidad de esos compuestos a través de la distribución natural del complejo, y para determinar su valor taxonómico. Con fines comparativos Agave lechuguilla y Agave striata se analizaron de la misma manera. Se encontró un total de 81 compuestos fenólicos, que incluyeron 18 ácidos fenólicos, 51 flavonoides (26 flavonoles, 19 dihidroflavonoides y seis flavonas), además de 12 compuestos fenólicos no identificados. Cada población del complejo A. victoriae-reginae presentó una composición fenólica foliar diferente, y A. pintilla, la especie más recientemente descrita del grupo, se pudo distinguir por cuatro compuestos no compartidos con ninguna otra población analizada. En todos los taxa se detectaron ácidos fenólicos; sin embargo, una predominancia de estos compuestos se encontró en el complejo A. victoriae-reginae, mientras que en A. striata se encontró predominancia de flavonoles (derivados de quercetina), y en A. lechuguilla una codominancia de dihidroflavonoides y flavonoles, lo que permitió discernir perfiles especie-específicos. Diferencias en los contenidos de algunos compuestos fenólicos entre estas tres especies y dentro del complejo A. victoriae-reginae también fueron encontradas. Los resultados son consistentes con la propuesta de considerar a A. victoriae-reginae como un complejo de especies y con la separación de A. pintilla como una especie independiente. Estos resultados revelan a los patrones fenólicos foliares como marcadores químicos valiosos en Agave.
Palabras clave: Agave lechuguilla, Agave striata, Agave victoriae-reginae, perfiles fenólicos, variabilidad química.
Agave victoriae-reginae T.Moore is one of the about 166 (Good-Ávila et al., 2006) to 200 (García-Mendoza, 2002) species of Agave. It is one of the most important ornamental species of the genus, belonging to subgenus Littaea (Gentry, 1982). Because its popularity as ornamental, some of its populations have become endangered due to illegal collections, and the Mexican government has listed the species as endangered to protect it (SEMARNAT, 2010). It has been considered as endemic to the Chihuahuan Desert of Mexico, with populations occurring in western Nuevo León, central Coahuila, and northeastern Durango (Eguiarte et al., 1999; Martínez-Palacios et al., 1999, 2003), as well as in southern Durango (González-Elizondo et al., 2009), outside the limits of the Chihuahuan Desert. High levels of genetic diversity within populations and of differentiation among populations of Agave victoriae-reginae were found by Martínez-Palacios et al. (1999), who reported that the levels of differentiation among some populations are comparable to those observed among subspecies or even species.
A recent taxonomic revision (González-Elizondo et al., 2011) reveals that what has been considered as A. victoriae-reginae represents a complex of three species, one with two subspecies: (1a) Agave victoriae-reginae subsp. victoriae-reginae (western Nuevo León and eastern Coahuila); (1b) A. victoriae-reginae subsp. swobodae J.J.Halda (southern Coahuila and northeastern Durango); (2) Agave nickelsiae Goss. ex Rol.-Goss. (microendemic to southeastern Coahuila); and (3) Agavepintilla S.González, M.González & L.Reséndiz, a newly described species (southeastern Durango). References to this complex from now on in this paper are given as A. victoriae-reginae s.l., whereas references to A. victoriae-reginae as a species are given without "s.l." or, when both cases are contrasted, as A. victoriae-reginae s. str.
Flavonoid profiles have been reported as worthy chemical markers with a species-specific tendency (Campos, 1997; Fiasson et al., 1997; Almaraz-Abarca et al., 2004; Almaraz-Abarca et al., 2006). In spite of the many taxonomic controversies about the delimitation of many species of Agave (Gentry, 1982; González-Elizondo et al., 2009), efforts focused in determining the taxonomic significance of the flavonoid profiles in this genus have been scarce. Agave americana (Parmar et al., 1992; Subramanian and Nair, 1970; Tinto et al., 2005), and A. barbadensis (Tinto et al., 2005) are among the few species of Agave studied for the flavonoid composition, although with no taxonomic aims. For those species, complex flavanoids were reported. One study focused on the taxonomic significance of the phenol profiles in Agave (Almaraz-Abarca et al., 2009), pointed out that foliar flavonoid profiles are valuable markers to discriminate among Agave asperrima, A. durangensis, A. shrevei subsp. shrevei, A. shrevei subsp. matapensis, and A. wocomahi.
In the present study, foliar flavonoid profiles of eight populations representing the total geographical distribution of Agave victoriae-reginae s.l. were obtained by high performance liquid chromatography with diode array detection (HPLC/DAD) in order to establish the variability among populations and the significance of these markers to support taxonomical proposals based on morphological traits. Agave lechuguilla Torr. and A. striata Zucc. were analyzed in the same manner as references and for identifying some accumulation tendencies of phenolic compounds in species of subgenus Littaea.
Materials and methods
Plant material. Foliar tissue of adult plants of 64 individuals from eight populations assumed as Agave victoriae-reginae, covering its natural distribution, was analyzed for their phenol composition by HPLC/DAD. With comparative purposes, ten individuals of Agave lechuguilla (series 2000) and an equal number of individuals of Agave striata (series 1000) were collected and analyzed in the same manner. Voucher specimens for each population sampled were deposited at Herbarium CIIDIR. The description of each sampling site and the number of individuals sampled for population and species are shown in Table 1.
Phenol extraction. Each sample was individually analyzed. Five grams of foliar dried grinded tissue were extracted by maceration for 24 h, in 25 mL 60% methanol (v/v), in darkness and at room temperature. The extracts were centrifuged (8,500 rpm) for 10 min at room temperature. The supernatants were separated and the pellets reextracted in 25 mL 60% methanol (v/v) by maceration for 3 h. The extracts were centrifuged at the same conditions. Similar superna-tants were brought together to form the total extracts; these were concentrated and fractionated with ethyl acetate before taking them to dryness under vacuum. Each organic fraction was then dissolved in 3 mL methanol; aliquots were taken to be used in the HPLC/DAD analyses.
HPLC/DAD analysis. The individual HPLC/DAD phenol profiles were obtained according to the method described by Campos (1997). Extracts (20 μL) were analyzed on a Perkin Elmer Series 200 HPLC system and Perkin Elmer Brownlee Analytical C18 column (4.6 x 250 mm, 5 μm), by an acidified acetonitrile-water gradient. The flow rate was 0.8 mL/min. Standard chromatograms were plotted at 280 and 340 nm. Spectral data for all peaks were accumulated in the range 200-400 nm using a diode-array detector (DAD) (Perkin Elmer Series 200). The structural information was obtained by direct comparison of retention times and UV spectra of the compounds found with those of standards, and according to the compilations done by Mabry et al. (1970) and Campos and Markham (2007). The contents of eight selected compounds, according to their presence only in both A. lechuguilla and A. striata (compounds 4 and 33), only in A. victoriae-reginae from El Mezquital (compounds 5 and 37), and only in most populations of A. victoriae-reginae s.l. (compounds 8, 11, 29, and 48) were estimated by calibration curves (5-100 μg mL-1 vs. peak area) constructed by linear regression via HPLC (peak areas) obtained from quercetin-3-O-rhamnoside (intercept = -0.00121, slope = 0.05849, r = 0.9963) for quercetin derivatives, and from gallic acid (intercept = 0.0184, slope = 0.0894, r = 0.9963) for derivatives of phenolic acids. The contents were expressed in μg g-1 dry weight. They were estimated and compared for samples collected in April-May (the population of Viesca was excluded because it was collected in August), and in April-May and August only for the samples of A. victoriae-reginae from El Mezquital.
Data analysis. The individual phenol profiles were made up of all compounds present in the respective HPLC/DAD chromatograms. Each compound was treated as a single chemical character. A binary matrix coded by 1 (presence) or 0 (absence) formed by all individual samples vs. all resolved compounds (84 individuals vs. 81 compounds) was analyzed using Principal Components Analysis (PCA) from Past 1.43 (Hammer et al., 2001).
Results
Foliar phenol composition. Eighty one compounds were detected for the 84 analyzed individuals. Their retention times and spectral data are shown in Table 2. Flavonoids (51 compounds) and phenolic acids (18 compounds) were the major phenols detected in the foliar tissue of Agave victoriae-reginae s.l., A. lechuguilla, and A. striata. Among the phenolic acids, nine were identified as benzoic acid derivatives and nine as cinnamic acid derivatives. Flavonols (26 compounds), flavones (6 compounds), and dihydroflavonoids (19 compounds) were the classes of flavonoids identified. Figure 1 shows the UV-spectra of compounds representing the different classes of phenols detected in the foliar tissue of A. victoriae-reginae s.l., A. lechuguilla, and A. striata.
Kaempferol (a flavonol aglycone, compound 68) was detected in Agave victoriae-reginae of Bustamante, Nuevo León; its UV-spectrum is shown in Figure 2. The foliar phenol profiles of the different A. victoriae-reginae s.l. populations, A. striata, and A. lechuguilla are shown in Table 3.

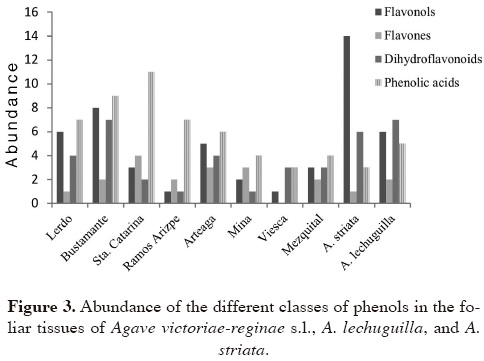
Taxonomic significance. The abundance of each type of phenolic compound found in the foliar tissue of Agave victoriae-reginae s.l., A. striata, and A. lechuguilla are shown in Figure 3. Variations in the accumulation levels of compounds can be observed in the different taxa. The levels of eight compounds (4, 5, 8, 11, 29, 33, 37, and 48) present in the taxa analyzed are displayed in Table 4. Some variations among taxa and between two seasons in the same taxon (A. victoriae-reginae s.l. from El Mezquital) are observed.
The PCA based in a matrix constructed with all the compounds resolved in the HPLC/DAD chromatograms vs. all the individuals analyzed, revealed the separation of Agave striata from A. lechuguilla and A. victoriae-reginae s.l. (Figure 4). The separation of A. lechuguilla, A. victoriae-reginae s.l. from El Mezquital, and all other populations of A. victoriae-reginae s.l., is observed in the same Figure 4, agreeing with the fact that they are independent species.
Discussion
Foliar phenol composition. In order to obtain fingerprinting chromatograms, the HPLC analysis were done with the unhydrolyzed extracts. The HPLC/DAD is considered as a highly effective technique for the separation and for obtaining structural information of phenolic acids and flavonoids (Markham and Bloor, 1998).
The presence of kaempferol (compound 68) in Agave victoriae-reginae of Bustamante, Nuevo León, one of the most arid sites among the studied here, and the report about flavonoid aglycones being most often produced and accumulated externally in plants from arid and semiarid habitats (Wollenweber, 1994) suggest that the species here analyzed may synthesize more flavonoid aglycones, but they could not be detected because polar solvents were used in the extract preparations, which preferentially dissolve flavonoid glycosides. Even so, the identification of 25 flavonol glycosides in the taxa of Agave here analyzed indicate that these species of plants from arid and semiarid habitats can synthesize and accumulate a relatively high number of flavonoid glycosides. The screening of flavonoid aglycones in those species would deserve further investigation for exploring their taxonomic significance, as well as for the role that those compounds play in protecting the plant tissues from damaging UV-B (280-315 nm) radiation (Williams and Harborne, 1994), and for the pharmacological properties those compounds show (Wollenweber, 1994). Quercetin-O-glycoside derivatives were the most abundant (18 compounds) among the flavonols. Derivatives of herbacetin (compound 31 ), and isorhamnetin derivative (compound 69) were other flavonols also found.
A complex flavanone (5,7dihydroxi-6,5'-di-metoxi-3', 4'-metilenedioxiflavanone) present in somatic tissues (Parmar et al., 1992), and of two flavonol glycosides (kaempferol-3-O-glucoside and kaempferol-3-O-rutinoside) from the flowers of A. americana (Subramanian and Nair, 1970) have been reported. Also, three complex isoflavanoids have been identified in the rhizomes of A. barbadensis (Tinto et al., 2005). Twenty three flavonoids were recently reported by Almaraz-Abarca et al. (2009) for adult foliar tissues of A. durangensis, all flavonol glycosides, most of them being derivatives of kaempferol. Those last authors also reported three kaempferol glycosides, as unique components of the phenolic profile for Agave asperrima. Flavonols, one of the three classes of flavonoids previously reported in species of Agave (flavanones, flavonols and isoflavonoids) were also found in the present study for A. victoriae-reginae s.l., A. lechuguilla, and A. striata, and contrary to those former reports, phenolic acids derivatives, flavones, dihydroflavonoids, and a higher diversity of flavonols, including derivatives of herbacetin, and ishoramnetin were detected (Figure 1, Table 2). Phenolic acids provide the precursors for lignin, tannins, and flavonoid biosynthesis (Hrazdina, 1992; Heller and Forkmann, 1994). in species of Agave, which do not accumulate relevant amounts of foliar lignin neither tannins (Wall et al., 1957; Seigler, 2002), phenolic acids could have several other functions, like protection against herbivores and pathogens, action as cementing material joining phenolic polymers to polysaccharides of cell walls (Wallace and Fry, 1994), participation in the regulation of cell growth and division (Binns et al., 1987), inhibition of ion intake by a generalized increase in membrane permeability to inorganic ions as a mode of action of allelopathic compounds (Glass and Dunlop, 1974), protection to thermal stress (Dixon and Paiva, 1995), and function as antioxidants (Rice-Evans et al., 1997; Almaraz-Abarca et al., 2007), which could give survival advantages to A. victoriae-reginae s.l., A. lechuguilla, and A. striata, that synthesize those phenols.
Taxonomic significance. Fifty one phenols were found by HPLC/DAD in the foliar tissue of Agave victoriae-reginae s.l. (considering the eight populations). From those, 45 were found in A. victoriae-reginae s. str., excluding the populations of Ramos Arizpe and Arteaga, which correspond to A. nickelsiae, and the population of El Mezquital, which corresponds to A. pintilla, according to González-Elizondo et al. (2011). For A. striata were detected 24, and also 24 (most of them different) in A. lechuguilla (Table 3). This distribution of phenols indicates that the group most diverse, regarding these secondary metabolites, is that of A. victoriae-reginae. That chemical richness is the highest reported yet for any species of Agave if compared with the reports of Subramanian and Nair (1970), and Parmar et al. (1992) for A. americana; Tinto et al. (2005) for A. barbadensis; Chen et al. (2009) for A. sisalana; and Almaraz-Abarca et al. (2009) for A. asperrima, A. durangensis, A. shrevei subsp. shrevei, A. shrevei subsp. matapensis, and A. wocomahi. Nineteen (compounds 6, 12, 17, 18, 26, 28, 31, 34, 40, 42, 44, 45, 49, 50, 58, 61, 63, 70, and 78) among the 24 phenols forming the profile of A. striata were unique to this species, and six of them (compounds 34, 42, 45, 50, 70, and 78) were present in every analyzed individual of this taxon; while seven (compounds 2, 14, 54, 71, 79, 80, and 81) among the 24 phenols detected for A. lechuguilla were only present in this species (Table 2). A higher number, thirty seven (compounds 3, 5, 8, 10, 11, 13, 16, 19, 22, 23, 24, 27, 29, 30, 32, 37, 38, 41, 43, 47, 48, 51, 53, 55, 56, 57, 60, 62, 64, 66, 67, 68, 69, 72, 75, 76, and 77) among 51 phenols, was detected only in A. victoriae-reginae s.l. (Table 2). The predominance of phenolic acids in A. victoriae-reginae s.l. and of flavonols, majorly quercetin-o-glycosides, in A. striata, can be observed in Figure 3. Agave striata was collected at the same location than one of the populations of A. victoriae-reginae, at Lerdo, Durango (Table 1), where they are sympatric, exposed to the same environmental conditions. This allows to conclude that the evident differences in their foliar phenol profiles are the result of genetic differences that command species-specific sequential order in the biosynthesis pathway, as Heller and Forkmann (1994) pointed out, of which many of individual steps of the flavonoid modification have shown to be controlled by single genes (Forkmann, 1994). our results indicate a species-specific tendency of phenol profiles for those taxa, as has been found for other groups of plants (Bate-Smith et al., 1975; Campos, 1997; Almaraz-Abarca et al., 2006).
The remarkable differences in the phenol profiles between Agave victoriae-reginae s.l. and A. striata correspond to the fact that the former belongs to a different series (Marginatae) than A. striata, which belongs to the series Striatae (Gentry, 1982). And although Agave lechuguilla is closer to A. victoriae-reginae s.l. (both belonging to the series Marginatae), the differences in the phenol profiles can also be observed between them (Table 3). in both, A. lechuguilla and A. victoriae-reginae s.l., flavonols are not predominant, but in A. lechuguilla a codominance of flavonols and dihydroflavonoids was detected, unlike A. victoriae-reginae s.l., in which phenolic acids are abundant (Figure 2). Agave lechuguilla grows also in Lerdo, Durango, under the same environmental conditions that A. victoriae-reginae and A. striata, so that differences in the phenolic profiles can be also assumed as specific expression patterns.
Although flavonoids are the major chemotaxonomic markers (Markham, 1982; Harborne and Turner, 1984), phenolic acids also possess a certain value as chemical markers (Van Sumere, 1989), and may be ubiquitous and accumulated at significant levels in plant tissues (Tamagnone et al., 1998). Pollen of some plant species may be particularly rich in phenolic acids (Campos, 1997; Almaraz-Abarca et al., 2004; Almaraz-Abarca et al., 2007) but these may be completely missing in the pollen of others, as in A. durangensis (Almaraz-Abarca et al., 2009). To our knowledge, the ubiquitous richness and diversity of benzoic acid and cinnamic acid derivatives in the foliar tissue of Agave, particularly of A. victoriae-reginae s.l. and A. lechuguilla, are reported for the first time.
The presence of phenolic acids in the species here studied, all of them belonging to Agave subgenus Littaea, contrasts with their absence in A. americana (Parmar et al., 1992), A. asperrima, A. durangensis, A. shrevei, A. wocomahi, (Almaraz-Abarca et al., 2009), and A. sisalana (Chen et al., 2009), which belong to Agave subgenus Agave. if those tendencies are found in more species of both subgenera, it could be suggested that the relative abundance of phenolic acids may have some taxonomic relevance at the subgeneric level in Agave. The trend of a taxon to synthesize preferentially one phenol type rather than other one could be an indicator of evolutionary tendencies within Agavaceae, as has been established for Asteraceae by Emerenciano et al. (2001), among other many dicotyledonous, and for monocotyledonous other than Agavaceae, e.g., for Juncaceae (Williams and Harborne, 1975), Liliaceae (Williams, 1975), Fluviales (Harborne and Williams, 1976a), Gramineae (Harborne and Williams, 1976b), and orchidaceae (Williams, 1979). To establish the evolutionary connotation of the flavonoid accumulation in this group is necessary to analyze more taxa of monocots, since some differences have been detected with respect to the tendencies found in dicotyledons, as has been pointed out by Harborne (1977).
The species of Agave here analyzed showed some chemical characters reported by Salatino et al. (2000) as primitive in Ericaulaceae (a monocotyledonous family), such as complex patterns of flavonoids (formed by several different types of phenols). Some chemical features, such as the accumulation of methylated flavonoids, considered as evolved for Pinus (Kaundun and Lebreton, 2010), were found in this study (compound 69, an isorhamnetin derivative) for plants from Lerdo, Bustamante, and Viesca, all belonging to A. victoriae-reginae s.str. To establish the evolutionary tendency of the accumulation of phenolic compounds in Agavaceae, and particularly in Agave, more detailed analysis on a broader number of species are needed.
Martínez-Palacios et al. (1999) mentioned for Agave victoriae-reginae high levels of genetic variability within populations as well as high levels of interpopulation differentiation. This variability is also observed in the foliar phenol profiles exhibited by the different populations here analyzed (Table 3, Figures 3, 4). Six among the eight populations of A. victoriae-reginae s.l. (Santa Catarina, El Mezquital, Arteaga, Mina, Lerdo, and Bustamante) displayed one to six unique compounds, not found in any other. Thus, compound 22 was found only in the population of Mina; compounds 41 and 55 were detected in Lerdo; compounds 56 and 68 in Bustamante; compounds 10 and 35 in Arteaga; compounds 5, 16, 37, and 77 in El Mezquital; and compounds 3, 13, 21, 27, 57, and 62 in Santa Catarina.
This distribution of phenol profiles corresponds partially to the separation of taxa based on morphological traits. The population of El Mezquital, the most isolated geographically, which corresponds to a different species, namely A. pintilla (González-Elizondo et al., 2011), is distinguished by possessing four unique compounds. But phenol profiles did not allow to distinguish between Agave victoriae-reginae s. str. and A. nickelsiae, as found by Martínez-Palacios et al. (1999) using allozymes. in the Figure 4, the eight populations of Agave victoriae-reginae s.l. formed four groups: (A) Viesca; (B) Arteaga with some individuals from Bustamante, and Lerdo; (C) Mina, Santa Catarina, Ramos Arizpe, and some individuals from Bustamante; and (D) El Mezquital. The separation of all the individuals of El Mezquital from the rest is consistent with the proposal, based on morphological attributes, of non conspecificity for this population, and of considering to A. victoriae-reginae as a complex instead of a single species (González-Elizondo et al., 2011). However, some inconsistencies were found among our chemical results and the ones reported by González-Elizondo et al. (2011) based on morphological traits and by Martínez-Palacios et al. (1999) based on allozymes. Excluding the population of El Mezquital, which was not analyzed by Martínez-Palacios et al. (1999), the three other groups (Figure 4) did not clearly correspond to the groups proposed by either González-Elizondo et al. (2011) or Martínez-Palacios et al. (1999), except for group (C) (Figure 4), which roughly corresponds to the eastern populations found by Martínez-Palacios et al. (1999). The incongruence between our results and those reported by those authors are comparable to those reported between morphological and molecular features for other species of Agave (Alfaro et al., 2007). incongruence between different kinds of markers has been explained by Hörandl (2010) as a result of an asynchronous marker evolution.
The foliar tissues of Agave victoriae-reginae s.l., A. lechuguilla, and A. striata have complex profiles of phenols, which are rich in number and classes of those compounds, including phenolic acids, flavonols, flavones, and dihydroflavonoids. The three taxa can be distinguished by the different qualitative and quantitative phenol patterns. The presence of unique phenols in the different populations of A. victoriae-reginae s.l. suggests the recognition of four chemotypes in that variable group. Phenol composition supports the proposal of considering the population of El Mezquital as an independent species (A. pintilla).
Quantitative variations. Variations in the amount accumulated of some compounds could be detected among the different populations of Agave victoriae-reginae s.l., A. lechuguilla, and A. striata (Table 4). Concerning the levels of the compounds 8, 11, 29, and 48, the populations of Lerdo, Bustamante, Santa Catarina, Ramos Arizpe, Arteaga, and Mina (all sampled in May) showed different levels of them, suggesting that those variations depend on the geographical origin. The difference observed in compound 33, a flavone (Table 4), common to A. lechuguilla and A. striata, collected at the same season and the same location, suggests that important variation can be found between species synthesizing the same compound. Considerable variation in concentrations were observed in the compounds 5 and 37 (unidentified), both in the population of El Mezquital (A. pintilla) between individuals sampled in May-June and those sampled in October (Table 4), with an increased concentration in october with respect to May-June, suggesting fluctuations correlated to seasonal changes in adult plants of Agave. This increase in concentration could be associated to a response to the increase of herbivores and pathogenic fungi and bacteria attack due to an increase of the precipitation, since phenols are known to be active against those organisms (Echeverri et al., 1991; Hadacek, 2002).
Due to their stability, qualitative phenolic profiles have more taxonomic significance than quantitative patterns (Harborne and Turner, 1984); however, further investigation needs to be performed to determine the fluctuation patterns associated to different environmental conditions and also to developmental stages of the accumulated phenolics in Agave, not only with taxonomic aims but also in order to extend our knowledge on the role and synthesis regulation of those compounds in the genus.
Acknowledgements
The authors appreciate the help of Martha González Elizondo, Lorenzo Rezéndiz Rojas, and Lorena López Enríquez to solve taxonomical issues and in part of the field work. The authors also thanks the Comisión de Fomento a las Actividades Académicas del Instituto Politécnico Nacional (COFAA IPN) for stimuli for research, and the reviewers, who made suggestions that improved the quality of the manuscript.
Literature cited
Alfaro R.G., Legaria S.J.P and Rodríguez P.J.E. 2007. Diversidad genética en poblaciones de agaves pulqueros (Agave spp.) del nororiente del Estado de México. Revista Fitotecnia Mexicana 30:1-12. [ Links ]
Almaraz-Abarca N., Campos M.G., Ávila-Reyes J.A., Naranjo-Jiménez N., Herrera-Corral J. and González-Valdez L.S. 2004. Variability of antioxidant activity among honeybee-collected pollen of different botanical origin. Interciencia 29:574-578. [ Links ]
Almaraz-Abarca N., González-Elizondo M.S., Tena-Flores J.A., Ávila-Reyes J.A., Herrera-Corral J. and Naranjo-Jiménez N. 2006. Foliar flavonoids distinguish Pinus leiophylla and Pinus chihuahuana (Coniferales: Pinaceae). Proceedings of the Biological Society of Washington 119:426-436. [ Links ]
Almaraz-Abarca N., Campos M.G., Ávila-Reyes J.A., Naranjo-Jiménez N., Herrera-Corral J. and González-Valdez L.S. 2007. Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). Journal of Food Composition and Analysis 20:119-124. [ Links ]
Almaraz-Abarca N., Delgado-Alvarado E.A., Hernández-Vargas V., Ortega-Chávez M., Orea-Lara G., Cifuentes-Díaz de León A. , Ávila-Reyes J.A. and Muñiz-Martínez R. 2009. Profiling of phenolic compounds of somatic and reproductive tissues of Agave durangensis Gentry (Agavaceae). American Journal of Applied Sciences 6:1076-1085. [ Links ]
Bate-Smith E.C., Ferguson I.K., Hutson K., Jensen S.R., Nielsen B. J. and Swain T. 1975. Phytochemical interrelationships in the Cornaceae. Biochemical Systematics and Ecology 3:79-89. [ Links ]
Binns A.N., Chen R.H., Wood H.N. and Lynn D.G. 1987. Cell division promoting activity of naturally occurring dehydrodi-coniferyl glucosides: do cell wall components control cell division? Proceedings of the National Academy of Sciences USA 84:980-984. [ Links ]
Campos M.G.R. 1997. Caracterização do pólen apícola pelo seu perfil em compostos fenólicos e pesquisa de algumas actividades biológicas. Tesis de Doutoramento, Faculdade de Farmácia, Universidade de Coimbra. Portugal, 318 pp. [ Links ]
Campos M.G. and Markham K.R. 2007. Structure Information from HPLc and on-line Measured Absorption Spectra: Flavo-nes, Flavonols and Phenolic Acids. impresa da Universidade de Coimbra, Coimbra. [ Links ]
Chen PY., Kuo Y.C., Chen C.H., Kuo Y.H. and Lee C.K. 2009. Isolation and immunomodulatory effect of homoisoflavones and flavones from Agave sisalana Perrine ex Engelm. Molecules 14:1789-1795. [ Links ]
Dixon R.A. and Paiva N.L. 1995. Stress-induced phenylpropanoid metabolism. The Plant Cell 7:1085-1097. [ Links ]
Eguiarte L.E., Larson-Guerra J., Nuñez-Farfán J., Martínez-Palacios A., Santos del Prado K. and Arita H.T. 1999. Diversidad filogenética y conservación: ejemplos a diferentes escalas y una propuesta a nivel poblacional para Agave victoriae-reginae en el desierto de Chihuahua, México. Revista Chilena de Historia Natural 72:475-492. [ Links ]
Echeverri F., Cardona G., Torres F., Pelaez C., Quiñones W. and Rentería E. 1991. Ermanin: an insect deterrent flavonoid from Passiflora foetida resin. Phytochemistry 30:153-155. [ Links ]
Emerenciano V.P., Militão J.S.L.T., Campos C.C., Romoff P., Kaplan M.A.C., Zambon M. and Brant A.J.C. 2001. Flavonoids as chemotaxonomic markers for Asteraceae. Biochemical Systematics and Ecology 29:947-957. [ Links ]
Fiasson J.L., Gluchoff-Fiasson K. and Dahlgren G. 1997. Flavonoid patterns in European Ranunculus L. subgenus Batrachium (Ranunculaceae). Biochemical Systematics and Ecology 25:327-333. [ Links ]
Forkmann G. 1994. Genetics of flavonoids. In: Harborne J.B. Ed. The Flavonoids. Advances in Research since 1986, pp. 537-564, Chapman and Hall, London. [ Links ]
García-Mendoza A. 2002. Distribution of the genus Agave (Agavaceae) and its endemic species in Mexico. Cactus and Succulent Journal 74:177-187. [ Links ]
Gentry H. S. 1982. Agaves of Continental North America. University of Arizona Press, Tucson. [ Links ]
Glass A.D.M. and Dunlop J. 1974. Influence of phenolic acids on ion uptake: IV. Depolarization of membrane potentials. Plant Physiology 54:855-858. [ Links ]
González-Elizondo M., Galván-Villanueva R., López-Enríquez I.L., Reséndiz-Rojas L. and González-Elizondo M.S. 2009. Agaves. Magueyes, Lechuguillas y Noas del Estado de Durango y sus Alrededores. IPN-CONABIO-COCYTED, Durango. [ Links ]
González-Elizondo M.S., González-Elizondo M., López-Enriquez I.L., Reséndiz-Rojas L., Tena-Flores J.A. and Retana-Rentería F.i. 2011. El Complejo Agave victoriae-reginae (Agavaceae). Acta Botanica Mexicana 95:65-94. [ Links ]
Good-Ávila S.V., Souza V., Gaut B.S. and Eguiarte L.E. 2006. Timing and rate of speciation in Agave (Agavaceae). Proceedings of the National Academy of Sciences USA 103:9124-9129. [ Links ]
Hadacek F. 2002. Secondary metabolites as plant traits: current assessment and future perspectives. Critical Reviews in Plant Sciences 21:273-322. [ Links ]
Hammer Ø., Harper D.A.T. and Ryan PD. 2001. PAST: Paleonto-logical statistics software package for education and data analysis. Palaeontologia Electronica 4:4. [ Links ]
Harborne J.B. 1977. Flavonoids and the evolution of the angiosperms. Biochemical Systematics and Ecology 5:7-22. [ Links ]
Harborne J.B. and Turner B.L. 1984. Plant Chemosystematics. Academic Press, London. [ Links ]
Harborne J.B. and Williams C.A. 1976a. Occurrence of sulphated flavones and caffeic acid esters in members of the Fluviales. Biochemical Systematics and Ecology 4:37-41. [ Links ]
Harborne J.B. and Williams C.A. 1976b. Flavonoid patterns in leaves of the Gramineae. Biochemical Systematics and Ecology 4:267-280. [ Links ]
Heller W. and Forkmann G. 1994. Biosynthesis of flavonoids. In: Harborne J.B. Ed. The Flavonoids. Advances in Research since 1986, pp. 499-535, Chapman and Hall, London. [ Links ]
Hörandl E. 2010. Beyond cladistics: extending evolutionary classification into deeper time levels. Taxon 59:345-350. [ Links ]
Hrazdina G. 1992. Biosynthesis of flavonoids. In: Hemingway R.W. and Laks P.E. Eds. Plant Polyphenols Synthesis, Properties and Significance, pp. 61-72, Plenum Press, New York. [ Links ]
Kaundun S.S. and Lebreton P 2010. Taxonomy and systematic of the genus Pinus based on morphological, biogeographical and biochemical characters. Plant Systematics and Evolution 284:1-15 [ Links ]
Mabry T.J., Markham K.R. and Thomas M.B. 1970. The Systematic Identification of Flavonoids. Springer-Verlag, New York. [ Links ]
Markham K.R. 1982. Techniques of Flavonoid Identification. Academic Press, London. [ Links ]
Markham K.R. and Bloor S.J. 1998. Analysis and identification of flavonoids in practice. In: Rice-Evans C.A. and Packer L. Eds. Flavonoids in Health and Disease, pp. 1-33, Marcel Dekker, New York. [ Links ]
Martínez-Palacios A., Eguiarte L.E. and Furnier G.R. 1999. Genetic diversity of the endangered endemic Agave victoriae-reginae (Agavaceae) in the Chihuahuan Desert. American Journal of Botany 86:1093-1098. [ Links ]
Martínez-Palacios A., Ortega-Larrocea M.P., Chávez V.M. and Bye R. 2003. Somatic embryogenesis and organogenesis of Agave victoriae-reginae: Considerations for its conservation. Plant Cell, Tissue and organ Culture 74:135-142. [ Links ]
Parmar VS., Jha H.N., Gupta A.K. and Prasad A.K. 1992. Agamanone, a flavanone from Agave americana. Phytochemistry 31:2567-2568. [ Links ]
Rice-Evans C., Miller N. and Papanga G. 1997. Antioxidant properties of phenolic compounds. Trends in Plant Science 2:152-159. [ Links ]
Salatino A., Salatino M.L.F., dos Santos D.Y.A.C. and Patrício M.C.B. 2000. Distribution and evolution of secondary metabolites in Eriocaulaceae, Lythraceae and Velloziaceae from "campos rupestres". Genetics and Molecular Biology 23:931-940. [ Links ]
SEMARNAT. Secretaría de Medio Ambiente y Recursos Naturales. 2010. Norma Oficial Mexicana NOM-059-ECOL-2010. Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Secretaría de Medio Ambiente y Recursos Naturales. México, D.F [ Links ]
Seigler D.S. 2002. Plant Secondary Metabolism. Kluwer Academic Publishers, Boston. [ Links ]
Subramanian S.S. and Nair A.G.R. 1970. Chlorogenin and kaem-pferol glycosides from the flowers of Agave americana. Phytochemistry 9:2582. [ Links ]
Tamagnone L., Merida A., Stacey N., Plaskitt K., Parr A., Chang C. F., Lynn D., Dow M., Roberts K. and Martin C. 1998. Inhibition of phenolic acid metabolism results in precocious cell death and altered cell morphology in leaves of transgenic tobacco plants. The Plant Cell 10:1801-1816. [ Links ]
Tinto W.F., Simmons-Boyce J.L., McLean S. and Reynolds W.F. 2005. Constituents of Agave americana and Agave barbadensis. Fitoterapia 76:594-597. [ Links ]
Van Sumere C.F. 1989. Phenols and phenolic acids. In: Harborne J.B. and Dey P.M. Eds. Methods in Plant Biochemistry vol. 1, pp. 29-73, Academic Press, London. [ Links ]
Wall M.E., Fenske C.S., Kenney H.E., Willaman J.J., Correll D. S., Schubert B.G. and Gentry H.S. 1957. Steroidal sapogenins XLIII. Survey of plants for steroidal sapogenins and other constituents. Journal of the American Pharmacists Association 46:653-684. [ Links ]
Wallace G. and Fry S.C. 1994. Phenolic components of the plant cell. International Review of Cytology 151:229-267. [ Links ]
Williams C.A. 1975. Biosystematics of the Monocotyledoneae-flavonoid patterns in leaves of the Liliaceae. Biochemical Systematics and Ecology 3:229-244. [ Links ]
Williams C.A. 1979. The leaf flavonoids of the Orchidaceae. Phytochemistry 18:803-813. [ Links ]
Williams C.A. and Harborne J.B. 1975. Luteolin and daphnetin derivatives in the Juncaceae and their systematic significance. Biochemical Systematics and Ecology 3:181-190. [ Links ]
Williams C.A. and Harborne J.B. 1994. Flavone and flavonol glycosides. in: Harborne J.B. Ed. The Flavonoids. Advances in Research since 1986, pp. 337-385, Chapman and Hall, London. [ Links ]
Wollenweber E. 1994. Flavones and flavonols. In: Harborne J.B. Ed. The Flavonoids. Advances in Research since 1986, pp. 259-335, Chapman and Hall, London. [ Links ]














