Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Botanical Sciences
versão On-line ISSN 2007-4476versão impressa ISSN 2007-4298
Bot. sci vol.91 no.2 México Jun. 2013
Ecología
Propagation of three Bursera species from cuttings
Propagación de tres especies de Bursera a partir de estacas
Carolina Castellanos-Castro1,2 and Consuelo Bonfil1,3
1 Departamento de Ecología y Recursos Naturales, Facultad de Ciencias, Universidad Nacional Autónoma de México, México, D. F., México.
2 Present address: School of Applied Science, Bournemouth University, Poole, Dorset, United Kingdom.
3 Author for correspondence: cbonfil@ciencias.unam.mx
Received: April 12th, 2012
Accepted: September 3rd, 2012
Abstract
Vegetative propagation of three species of Bursera (B. glabrifolia, B. copallifera and B. linanoe) was studied. Cuttings were collected during the dry season, when trees were leafless and dormant. In a first trial, the effects of species, indol-butyric acid concentration (control, 4,000, and 9,000 ppm), and relative age of stockplants (young vs. mature trees) on rooting success were evaluated. Percentage of rooted cuttings and number of roots per cutting differed significantly among species, indol-butyric acid, and age of the stockplants. The three species studied showed increased rooting when indol-butyric acid was applied compared to the control, but sensitivity to applied indol-butyric acid varied relative to the type of cutting. Cuttings from young stockplants attained higher rooting percentages but were less responsive to indol-butyric acid application. In a second trial performed later in the dry season, one species (B. linanoe) showed a significant increase in rooting success. Overall, rooting percentages higher than 70% were attained when the sources of cuttings were young stockplants and when indol-butyric acid was applied. These results show that propagation of these species from cuttings is feasible. Rooted cuttings may be used for the restoration of degraded secondary tropical dry forests, where they are usually absent, for commercial plantations, or in agroforestry.
Keywords: Burseraceae; indolbutyric acid; restoration; rooting; tropical dry forest.
Resumen
Se estudió la propagación vegetativa de tres especies de Bursera (B. glabrifolia, B. copallifera y B. linanoe). Las estacas se recolectaron durante la temporada seca, cuando los árboles se encontraban en estado de reposo y sin hojas. En un primer ensayo se evaluó el efecto de la especie, la concentración de ácido indol-butírico (control, 4,000 y 9,000 ppm) y la edad relativa de las plantas de origen (árboles jóvenes vs. maduros) en el enraizamiento. El porcentaje de estacas con raíces y el número de raíces por estaca difirió significativamente entre especies y en respuesta a la concentración de ácido indol-butírico y la edad de las plantas de origen. El porcentaje de enraizamiento aumentó en todas las especies cuando se aplicó ácido indol-butírico, pero la sensibilidad al ácido indol-butírico varió con el tipo de estaca. Las que provinieron de plantas juveniles alcanzaron mayores porcentajes de enraizamiento y respondieron menos a la aplicación del ácido indol-butírico. En un segundo ensayo, realizado más tarde durante la temporada seca, se presentó un aumento considerable en el éxito de enraizamiento de una especie (B. linanoe). En conjunto, cuando se usaron estacas de árboles jóvenes y se aplicó ácido indol-butírico, se alcanzaron porcentajes de enraizamiento superiores a 70%. Estos resultados muestran que la propagación de estas especies a partir de estacas es posible. Las estacas enraizadas pueden usarse para la restauración de bosques tropicales secos degradados, en donde normalmente están ausentes, para establecer plantaciones comerciales o en agroforestería.
Keywords: ácido indolbutírico; bosque tropical seco; Burseraceae; enraizamiento; restauración.
There are around 100 tree species in the genus Bursera (Burseraceae), which has a broad distribution in America. Its highest diversity is found in Mexico, especially along the Pacific coast and the Balsas river basin. Bursera species are dioecious, deciduous trees and shrubs, often dominant or subdominant in many tropical dry forests (McVaugh and Rzedowski, 1965; Rzedowski et al., 2004). The fruit is a drupe with an orange (yellow to red) pericarp, and is consumed by a wide range of animals, including birds, which act as dispersal agents (Scott and Martin, 1984).
Knowledge on propagation techniques for native species of trees of the tropical dry forest is scarce, and it is becoming increasingly necessary to broaden the spectrum of species that can easily be propagated (Bonfil and Trejo, 2010), considering that extensive areas of these forests have been severely disturbed by frequent forest fires, cattle raising and wood extraction (Murphy and Lugo, 1986, Janzen, 1988, Trejo and Dirzo, 2000, Miles et al., 2006; Sánchez-Azofeifa and Portillo-Quintero, 2011). Bursera trees usually become important in late successional stages in Mexican tropical dry forests (Rzedowski and Kruse, 1979; Romero-Duque et al., 2007; Lebrija-Trejos et al., 2008; Williams-Linera et al., 2011), and therefore their propagation and reintroduction in disturbed sites could be an effective strategy to accelerate succession and restore them. Since Bursera trees are frequently used as living fences, their production in local nurseries could enhance the conservation value of agricultural landscapes. A few species have been overexploited because of their economic importance, resulting in significant population decline, and their propagation could promote the establishment of agroforestry plantations. This is the case for the three species whose vegetative propagation we studied: B. linanoe (La Llave) Rzedowski, Calderón & Medina, B. glabrifolia (H.B.K.) Engel., and B. copallifera (Sessé & Moc., ex D. C.) Bullock.
The wood and seeds of B. linanoe (commonly known as linaloe) are used in traditional woodcrafting, essential oil extraction and traditional medicine. As a consequence of over-harvesting, trees are either absent or scarce in the vicinity of the villages where traditional crafts are manufactured (Hersch-Martínez and Glass, 2006; Fuentes-López, 2009). This reduced availability is also found in the areas where alebrijes (a popular handcraft from Oaxaca) are made from the soft wood of B. glabrifolia (Purata et al., 2004; Hernández-Apolinar et al., 2006). Resin of B. copallifera is collected from natural populations and sold in many regional markets as copal, a kind of incense used in traditional and religious ceremonies (Linares and Bye, 2008). All these traditional uses have decimated natural populations, and thus it is becoming increasingly important to establish enrichment and agroforestry plantings to fulfill regional demand and restore degraded forests.
Several studies have shown poor seed germination in a number of Bursera species, and therefore plant production from seed is unreliable (Johnson, 1992; Maradiga et al., 2000; Andrés and Espinosa, 2002; Bonfil-Sanders et al., 2008). Vegetative propagation by cuttings is a low-tech option which could allow the production of large numbers of plants in a short period of time (Landis et al., 1999). Also, vegetatively propagated plants could keep the favorable features of the parent trees and they could be selected for their domestication as agroforestry crops (Simons and Leakey, 2004). In addition, the potential use of stakes and cuttings has been put forward as a promising tool for the restoration of tropical dry forests (Ray and Brown, 1995; Zahawi, 2005). Some Bursera species are propagated by local farmers using large stakes (~1.5 m long), not only for B. simaruba in Mexico and the Caribbean, but also for B. grandifolia, B. lancifolia, B. glabrifolia, and B. linanoe in many tropical dry forests areas of Mexico (Hersch-Martínez et al., 2004; Zahawi and Holl, 2009). However, the use of large branches limits not only the number of new plants that can be obtained, but is also relatively expensive and may have negative impacts on small populations of Bursera.
Preliminary studies on the propagation of several species of Bursera from cuttings showed differences among species in rooting ability, as well as response to indol-butyric acid (IBA), and suggested that a refinement of the technique could improve the success of plant propagation by cuttings (Bonfil-Sanders et al., 2007). Thus, the current investigation was aimed at evaluating the effect of: (a) species, (b) IBA concentration, and (c) age of the stockplants, on rooting success of cuttings of Bursera glabrifolia, B. copallifera, and B. linanoe collected during the dry season. This first trial identified appropriate experimental conditions, which were then used in a second trial to confirm its efficiency and to propagate larger numbers of plants to test their field performance (not reported in this paper, but see Castellanos-Castro and Bonfil, 2010).
Materials and methods
The research was carried out between March and August 2007. Cuttings were collected from leafless individuals in different localities of the state of Morelos (central Mexico), during March and May. Period of collection was chosen following traditional knowledge on vegetative propagation of these species from large tree branches, which are used for living fences. Shoot cuttings of Bursera copallifera and B. glabrifolia were collected from vigorous individuals at the archaeological site of Xochicalco (18° 48' N, 99° 17' W, altitude 1,200-1,350 m), while cuttings from B. linanoe were collected at Chimalacatlán, Sierra de Huautla (18° 27' N, 99° 5' W, altitude 1,000 m). Both sites are part of national protected areas having secondary tropical dry forests in which Bursera trees are dominant. Forests are not managed in Xochicalco, while in the area of Chimalacatlán seeds of B. linanoe are collected for oil extraction. The climate in the region is warm subhumid, with a rainy season in summer (June to october), and a dry season that usually extends from December to May (García, 1988); annual mean temperature is 22.9 °C in Xochicalco and 29.2 °C near Chimalacatlán, while average annual precipitation is 1,055 and 900 mm, respectively.
The first trial was performed on cuttings collected in March, and evaluated the effect of species, IBA concentration and relative age (i.e., maturity) of the stock trees on the rooting ability of cuttings. Individual source trees were growing in natural forests in the vicinity of agricultural or cattle grazing areas. They were categorized as mature reproductive trees (≥ 5 m tall and a well-defined single trunk) or young non-reproductive saplings (≤ 2 m tall with one or more basal branches). For each plant species, 75 cuttings were collected from five adult trees (15 per tree), and 27-45 cuttings were collected from three to five young trees or saplings (~9 per sapling). There were fewer cuttings from young stockplants of Bursera glabrifolia and B. copallifera than from mature trees due to their lower availability in the field. From this sample 25 mature and 8-15 young cuttings were randomly assigned to one of the following solutions: control (water), 4,000 ppm IBA, and 9,000 ppm IBA. The number of cuttings per stock individual was the same in all treatments. The 4,000 ppm solution was available in the market (name AIB 0.4% solution), while the 9,000 ppm solution was obtained by dissolving 35% IBA tablets (name Radix 35% TB) in water, as indicated by the manufacturer (Intercontinental Import Export, S. A. for both products). In a second trial, the protocol achieving the best results in March (IBA concentration + age of stockplants) was applied to 100 cuttings per species collected in the dry season, in May 2007, to confirm its efficiency and to propagate a larger number of plants to test their field performance (not reported in this paper). For this trial five stock individuals were used for B. linanoe and six for B. copallifera and B. glabrifolia. The source individuals used in each collection date were not the same, but in all cases the same number of cuttings per stock tree was assigned to each treatment, to reduce the possibility of an individual stockplant effect.
All cuttings were taken from lateral twigs (hardwood corresponding to the last two growing seasons) growing in full sun. As they were collected in the middle of the dry season, all plants were leafless and relative humidity was low. Twigs were collected in the morning to avoid high irradiance and heat; the basal and proximal ends of each twig were distinguished by different angles of cut to keep their polarity; they were tied in groups according to their stockplant. On the same day of collection the twigs were taken to the nursery (at Jardín Etnobotánico INAH, Cuernavaca, Morelos) in closed coolers, in order to keep them in shaded, fresh conditions. At the nursery the cuttings were pruned to their final size and measured (mean length ± s.d. = 25.1 ± 3.6 cm and mean basal diameter ± s.d. = 12.4 ± 3.2 mm; 2-3 nodes per cutting); no more than two cuttings were obtained from each twig; one from the apex and the next just below it. Two longitudinal, superficial cuts of ~2 cm long were made at the basal end of each cutting (just deep enough to take off the bark, approximately 2-3 mm), and they were immediately immersed in the IBA solution for 1 min, after which they were sown (~5 cm depth) in black polyethylene bags filled with a mixture of black soil and agrolite (1:1 V/V). The sieved organic soil (obtained from fertile oak forest areas in the region) was purchased from local markets, while the agrolite (purchased from nursery suppliers) is a substrate of mineral origin that increases porosity and drainage, favoring root development and avoiding waterlogging from irrigation.
The nursery walls and ceiling were covered with white plastic film and water was applied by an automatic intermittent mist system activated twice a day for ~5 min; additionally cuttings were watered manually when necessary. Temperature and humidity were not controlled and daily fluctuations were registered with a datalogger during the rooting period (Hobo H8 Pro Temp/Rh). Average temperature and humidity (± s.d.) from 00:00 to 8:00 h were 18.5 °C (± 0.3) and 93.9% (± 3.7), from 8:00 to 16:00 they were 30.6 °C (± 0.9) and 63.6% (± 6.6), and from 16:00 to 24:00 h they were 23.3 °C (± 0.9) and 78.2% (± 9.5). Maximum and minimum values during the rooting period were 43.9 °C and 14.5 °C for temperature, and 100% and 26% for humidity. Photosynthetically active radiation (PAR) on a clear summer day (between 12:00 - 14:00 h) inside the nursery was 303.25 μmol seg-1 m-2, 23% of the full radiation on a clear day.
In the first trial, bud and leaf development were registered during the rooting period (three months, from March to June) as an indirect measure of plant health and possible root development. Rooting response evaluations were undertaken 11 and 15 weeks after setting the cuttings into the rooting medium, in order to allow for root growth and avoid early uprooting, which can cause wilting (personal observation). The variables registered were presence of callus and roots, number of roots and number of leaves and leaf buds per cutting. Root data were obtained by splashing water on the rooting medium and gently removing remains of the medium from the roots. once the information was registered, the cuttings were returned to the bag. For the collection conducted in May, the number of roots and the diameter and length of the largest root per cutting were recorded in July and September 2007.
Data analysis. A generalized linear model was used to analyze the effects of species, age of stockplant, and IBA concentration on rooting of cuttings collected in March. As the response variable was the number of rooted and non-rooted cuttings, a binomial error and a logit link function were used. The effects of the same variables on the number of roots per cutting were analyzed by means of a factorial ANoVA, previously transforming data (log N+1) to achieve normality and homocedasticity. The relationship between final number of leaves + buds and number of roots in cuttings of each species was evaluated using Fisher exact test.
In cuttings collected in May, the number of roots per species was compared using a Kruskal-Wallis test, while length and diameter of the longest root were analyzed using one factor ANOVA and Tukey post-hoc tests when appropriate.
Results
Eleven weeks after the first experiment was set, cuttings of all three species had initiated roots and the proportions recorded did not increase four weeks later, at the second root assessment. Callus formation was frequent only in the control treatment: 19.4% of cuttings in Bursera glabrifolia, 37.5% in B. copallifera, and 40% in B. linanoe. Most of the cuttings that developed noticeable calli failed to produce roots by the time of the final evaluation.
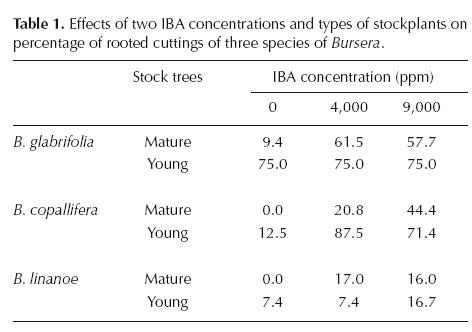
To analyze the proportion of rooted cuttings, a generalized linear model that included all main factors (species, type of cutting, and IBA concentration) and its interactions was initially used. However, as the fitting of this model was not significantly (P = 0.73) better than the one with only second grade interactions, the latter, simpler model was used for the analysis. All main factors had significant effects on the proportion of rooted cuttings: species (deviance 53.4, df 2, P < 0.0001), IBA concentration (deviance 42.4, df 2, P < 0.0001) and age of stockplant (deviance 15.6, df 1, P < 0.0001). The interaction age x IBA was also significant (deviance 8.37, df 2, P = 0.015), while remaining interactions were not (species x age P = 0.11 and species x IBA P = 0.18).
Bursera glabrifolia attained the highest mean rooting percentage (58.9% of all cuttings) and B. linanoe the lowest (9.2%). Higher proportions of rooted cuttings were observed when using IBA concentrations of 4,000 and 9,000 ppm than in the control (Table 1), and in cuttings from young trees than in those from mature trees. The significant interaction between age of stock tree and IBA is explained by a larger response to auxin application (i.e. increase in the proportion of rooted cuttings) in cuttings from mature trees than in those from younger trees. This was especially conspicuous in B. glabrifolia, in which there was no noticeable response to applied IBA when using young cuttings, but it was evident among cuttings from mature trees (Table 1).
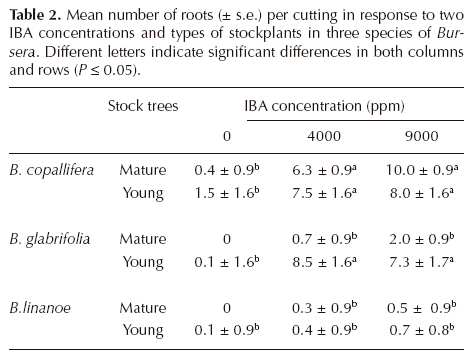
Root development. The effect of species (F = 54.9, df 2, P < 0.0001), age of stock tree (F = 21.2, df 1, P < 0.0001), applied IBA (F = 29.8, df 2, P < 0.0001), and the interactions species x age (F = 7.04, df 2, P = 0.0007), species x IBA (F = 6.2, df 4, P < 0.0001), and species x age x IBA (F = 2.7, df 4, P = 0.03) on number of roots per cutting were significant. Mean number of roots per cutting was largest in Bursera glabrifolia and smallest in B. linanoe (Table 2). In B. copallifera and especially in B. glabrifolia this number increased in cuttings derived from young stockplants relative to mature ones, but this difference was not quite as important in B. linanoe, which accounts for the significant interaction species x age. Not all species showed the same response to IBA application, as differences among cuttings in the control treatment and those with IBA were much larger in B. glabrifolia and B. copallifera than in B. linanoe. Overall, differences among cuttings derived from younger and mature stockplants were small without IBA (control), but increased when the auxin was applied, particularly in B. glabrifolia (Table 2).
A significant positive association was found between presence of buds and leaves, and the presence of roots in Bursera glabrifolia and B. copallifera (Fisher exact test P = 1.6 x 10-17 and P = 2.2 x 10-14 respectively). These observations allowed us to establish that cuttings with the best root development were also the ones that had the largest number of leaves, while the ones that did not develop leaves usually had no roots, though they could have a few leaf buds. Buds were recorded in a high proportion of cuttings 11 days after sowing, while fully expanded leaves were observed ten days later. overall, correlations between the initial size (length and diameter) of the cuttings and the number of roots per cutting were not significant in B. glabrifolia nor in B. copallifera, except for length of the cutting in the former species (r = 0.37, P = 0.005). The low number of rooted cuttings of B. linanoe precluded these analyses.
Although it was not our main goal to evaluate differences in rooting ability in cuttings obtained from different individual source trees, we noticed that most trees produced high proportions of rooted cuttings, but in one or a few of them this proportion was much lower; this pattern was observed in all species and in cuttings of both ages.
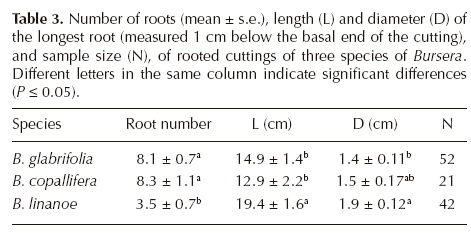
In the second collection made later in the dry season (in May, approximately eight weeks later), only the IBA concentrations producing the best results in cuttings from young trees were tried: 9,000 ppm in Bursera linanoe and 4,000 ppm in B. glabrifolia and B. copallifera. Rooting percentages were high and similar to those obtained in March for two species: B. glabrifolia 75.8% (cuttings from young stockplants in March 75%, see Table 1) and B. copallifera 70.6% (71-87% in March, Table 1), but in B. linanoe there was a noticeable increase in the percentage of rooted cuttings (81.4% in May vs. 16.7% in March). Comparisons among species on the number of roots per cutting and length and diameter of the longest root showed significant differences among species in the three variables (number of roots Kruskal-Wallis H = 26.7, P < 0.001; length and diameter of the largest root F = 3.69, df 2, P = 0.04 and F = 4.05, df 2, P = 0.02 respectively). Bursera glabrifolia and B. copallifera had more roots per cutting than B. linanoe, but the latter had a larger main root than the other two species (Table 3).
Discussion
Root development was successful in cuttings of the three Bursera species studied, and it was strongly influenced by IBA treatment and age of stockplants. The great majority of rooted cuttings developed buds, leaves, and roots by the time of the first evaluation, eleven weeks after sown, indicating that this period is appropriate to evaluate final root development under the temperature and humidity conditions found in the nursery.
There was no association between external callus and root formation. In cuttings of Bursera simaruba a high proportion of callus formation (0.93) and a low rooting proportion (0.04) has been reported (García-Orth, 2002), and in other Bursera species cuttings with conspicuous calluses frequently lack roots (Bonfil-Sanders et al., 2007). Although there is a general belief that callus tissue that develops as part of the wounding response is a precursor to the development of adventitious roots (Landis et al., 1999), it has been reported that recalcitrant species form callus without subsequent root formation (Hartmann et al., 1997), and a similar response might have occurred in untreated (control) cuttings. Callus formation without rooting has been reported to increase with ageing and related to unfavorable metabolic conditions for the development of roots (Husen and Pal, 2006).
A positive response of rooting to IBA application was observed in most cases, as in many other tropical species where this auxin induced root development, especially in leafy cuttings (Aminah et al., 1995; Mesén et al., 1997; Eganathan et al., 2000; Negash, 2002, 2003; Tchoundjeu et al., 2002; Husen and Pal, 2007a). No signs of toxicity due to the high concentrations of IBA used were observed, although negative effects on survival with similar and lower concentrations have been reported both on leafy and leafless cuttings (Ofori et al., 1996; Puri and Verma, 1996; Negash, 2002). However, full identification of optimal, suboptimal, and supraoptimal concentrations of the hormone will be achieved in future studies.
The higher root development found in cuttings from young rather than from mature trees has been reported in other species, such as Prosopis cineara (Arya et al., 1994), Inga feuillei (Brennan and Mudge, 1998), Juniperus procera (Negash, 2002), Argania spinosa (Nouaim et al., 2002), Robinia pseudoacacia and Grewia optiva (Swamy et al., 2002), Backhousia citriodora (Kibbler et al., 2004a) and Ulmus villosa (Bhardwaj and Mishra, 2005). Ontogenetic juvenility has been associated with a number of factors, such as optimum levels of auxins, sugars and carbohydrates, and low nitrogen levels (Bhardwaj and Mishra, 2005). Young plants also have less lignified tissues and lower production of rooting inhibitors than older plants (Leopold and Kriedemann, 1975, Hartmann et al., 1997). Other differences are related to higher peroxidase activity during root development in young cuttings (Husen and Pal, 2007b), and differences in fiber and vessel elements of the stem (Husen and Pal., 2006; Amissah et al., 2008).
In this study, juvenility refers to ontogenetic ageing, and differences in physiological juvenility were controlled by collecting young twigs (i.e., grown during the last two growing seasons) from both types of stockplants. It is not possible to establish which of the above mentioned factors associated with juvenility were more relevant in this case, but cuttings from young trees of all species have less lignified tissues than those from mature trees (as was evident by the different strength needed to collect them) and the bark is thinner in the former than in twigs from large reproductive trees. Endogenous auxin levels were probably not optimal even in cuttings from young trees, as they responded strongly to IBA application (except for Bursera glabrifolia). The significant interaction between age of stockplant and IBA also supports the hypothesis of metabolic differences between young and mature stockplants, as has been observed on cuttings of Dalbergia megaloxylon (Amri et al., 2010).
The young trees used as stock material were not as juvenile as smaller-sized or continuously hedged stockplants, but their morphology suggests that most of them had been coppiced and had resprouted. Additionally, these young trees allowed us to obtain more cuttings per individual than smaller, younger plants. The use of cuttings from young shoots on managed stockplants is recognized as the best vegetative propagation strategy (Landis et al., 1999; Negash, 2004), and future research in a larger set of Bursera species may help to establish the morphological and physiological characteristics of the shoots that encourage root development, as well as the best management strategy for the stockplants (Leakey, 2004).
Although the effect of different IBA concentrations on the percentage of rooted cuttings was analyzed using a small sample size in some cases (i.e., young cuttings of Bursera glabrifolia and B. copallifera, see methods section), the results were supported by the high percentage of rooted cuttings obtained later in the dry season, when the protocols rendering the best results in the first experiment were used. The only noticeable difference between results from these two trials was an increase in the proportion of rooted cuttings of B. linanoe in May. In species growing in highly seasonal environments, such as tropical dry forests, the increase in temperature during spring, when bud dormancy ends, induces carbohydrate mobilization and enzymatic activity, favoring root development in leafless hardwood cuttings (Puri and Verma, 1996; Bhardwaj and Mishra, 2005; Kibbler et al., 2004b, Haile et al., 2011). In this case, good results were obtained when leafless cuttings were completely dormant, prior to the beginning of bud swelling and development of flowers and leaves, which occurs early in May, before the onset of the rainy season, in B. copallifera and B. glabrifolia, (Velázquez-Herrera, 2011). Bursera linanoe grows in relatively drier conditions than the other two species (Hernández-Pérez et al., 2011), and buds may become active a few weeks later. In other tropical dry species an increase in rooting potential has also been found when cuttings are collected towards the end of the dry season but before bud breaking (Danthu et al., 2002; Haile et al., 2011).
When using the number of roots per cutting as a response variable to the experimental factors the same general trend was shown: all main factors had a very significant effect, as well as the interaction species x IBA. The positive effect of IBA on rooting percentage and on root number has been observed in other assessments of vegetative propagation (Amri et al., 2010), supporting its overall positive effect. We advise the use of young stockplants and IBA application when propagating cuttings of Bursera, considering differences regarding the best concentration for each species. It is worth noticing that even if young cuttings of B. glabrifolia did not need IBA to develop roots, the number of roots increased significantly when the hormone was applied to cuttings of both ages. A good root system is important for tree stability and growth (Asaah et al., 2010).
Shoot growth can reduce rooting as a result of internal competition for resources in leafy softwood cuttings (ofori et al., 1996; Mesén et al., 1997; Kibbler et al., 2004b), but a positive relationship between the development of both types of tissues is common in leafless hardwood cuttings and has been considered an indicator of metabolic activity (Tchigio and Duguma, 1998, Dick et al., 1998). The positive association found between bud and leaf development and root formation in two of three species of Bursera supports the second statement, and the fact that cuttings having the largest number of leaves were also the ones that developed more roots suggests the benefit of current assimilates to supplement the mobilization of stored carbohydrates in leafless cuttings from dormant shoots, and the absence of competition in this situation between the two types of tissues. Root development probably started a few weeks after the appearance of fully expanded leaves (three to four weeks), so a period of six weeks may be adequate to make an initial evaluation of root development and assess the speed of rooting in future studies.
It is likely that efforts to propagate other species of Bursera from young, dormant cuttings will be successful. Leafy cuttings can probably also be propagated under good propagation conditions, and a comparison of the rooting behavior of both types of cuttings will be useful to draw sound conclusions on the best procedure to propagate trees of this diverse and important genus. The propagation of large numbers of plants of Bursera species from small cuttings will certainly contribute to the restoration of degraded tropical dry forests of America and will also allow the establishment of productive plantations and agroforests in regions experiencing high demand of fruits, resin, or wood for scents and handcrafts.
Acknowledgements
C. Castellanos was supported by a scholarship from the Packard Foundation while doing this research. We thank P. Hersch, Ray and E. Soriano at the Jardín Etnobotánico of Instituto Nacional de Antropología e Historia for allowing us to use the nursery and other facilities. P. Mendoza, B. Barrales, F. García, A. Sierra, and R. Díaz helped with field and nursery work. This project was financially supported by the Universidad Nacional Autónoma de México through projects "Manejo de Ecosistemas y Desarrollo Humano" SDEIPTID-02 and PAPIIT- DGAPA IN218612. We thank Drs. R.R.B. Leakey, L. Negash, and one anonymous reviewer for their careful revision and comments to the original manuscript, which helped to improve it.
Literature cited
Aminah H., Dick J. McP., Leakey R.R.B., Grace J. and Smith R.I. 1995. Effect of indole butyric acid (IBA) on stem cuttings of Shorea leprosula. Forest Ecology and Management 72:199-206. [ Links ]
Amissah J.N., Paolillo D.J. and Bassuk N. 2008. Adventitious root formation in stem cuttings of Quercus bicolor and Quercus macrocarpa and its relationship to stem anatomy. Journal of the American Society for Horticultural Science 133:479-486. [ Links ]
Amri E., Lyaruu H.V.M., Nyomora A.S. and Kanyeka Z.L. 2010. Vegetative propagation of African Blackwood (Dalbergia melanoxylon Guill. & Perr.): effects of age of donor plant, IBA treatment and cutting position on rooting ability of stem cuttings. New Forests 39:183-194. [ Links ]
Andrés H.A.R and Espinosa O.D. 2002. Morfología de plántulas de Bursera Jacq. ex L. (Burseraceae) y sus implaciones filogenéticas. Boletín de la Sociedad Botánica de México 70:5-12. [ Links ]
Arya S., Tomar R. and Toky O.P. 1994. Effect of plant age and auxin treatment on rooting response in stem cuttings of Prosopis cineraria. Journal of Arid Environments 27:99-103. [ Links ]
Asaah E.K., Tchoundjeu T.N., Wanduku T.N and Van Damme P. 2010. Understanding structural roots system of 5-year-old Africam plum tree (D. edulis) of seed and vegetative origins (G. Don) H. J. Lam. Trees Structure and Function 24:789-796. [ Links ]
Bhardwaj D.R. and Mishra V.K. 2005. Vegetative propagation of ulmus villosa: effects of plant growth regulators, collection time, type of donor and position of the shoot on adventitious root formation in stem cuttings. New Forests 29:105-116. [ Links ]
Bonfil-Sanders C., Mendoza-Hernández P and Ulloa-Nieto J.A. 2007. Root and callus development in cuttings of seven species of the genus Bursera. Agrociencia 41:103-109. [ Links ]
Bonfil-Sanders C., Cajero-Lázaro I. and Evans R.Y. 2008. Seed germination of six Bursera species from central Mexico. Agrociencia 42:827-834. [ Links ]
Bonfil C. and Trejo I. 2010. Plant propagation and the ecological restoration of Mexican tropical deciduous forests. Ecological Restoration 28:369-376. [ Links ]
Brennan E.B. and Mudge K.W. 1998. Vegetative propagation of Inga feuillei from shoot cuttings and air layering. New Forests 15:37-51. [ Links ]
Castellanos-Castro C. and Bonfil S.C. 2010. Establecimiento y crecimiento inicial de estacas de tres especies de Bursera Jacq. ex L. Revista Mexicana de Ciencias Forestales 1:93-108. [ Links ]
Danthu P, Soloviev P., Gaye A., Sarr A., Seck M. and Thomas I. 2002. Vegetative propagation of some West Africa Ficus species by cuttings. Agroforestry Systems 55:57-63. [ Links ]
Dick J., Magingo F., Smith R.I. and McBeath C. 1998. Rooting ability of Leucaena leucocephala stem cuttings. Agroforestry Systems 42:149-157. [ Links ]
Eganathan P., Srinivasa Rao C. and Anand A. 2000. Vegetative propagation of three mangrove tree species by cuttings and air layering. Wetland Ecology Management 8:281-286. [ Links ]
Fuentes-López M.E. 2009. Comercialización de productos de linaloe. In: Solares-Arenas F. and Cruz-Cruz E. Ed. Ecología, Manejo Productivo y Comercialización del Linaloe (Bursera linanoe (La Llave) Rzedowski, Calderón & Medina), pp. 3549. INIFAP- Campo Experimental Valles Centrales de Oaxaca, México. [ Links ]
García E. 1988. Modificaciones al Sistema de Clasificación Climática de Köppen. Instituto de Geografía, Universidad Nacional Autónoma de México, México, D. F [ Links ]
García-Orth X. 2002. Efecto del ácido indolbutírico en la formación de callos y de raíces en estacas de Bursera simaruba (L.) Sarg., Gliricidia sepium (Jacq.) Kunth ex Walp. y Omphalea oleifera Hemsl., tres especies potencialmente útiles para restauración ecológica. Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, México, D. F. 66 pp. [ Links ]
Haile G., Gebrehiwot K., Lemenih M. and Bongers F. 2011. Time of collection and cutting sizes affect vegetative propagation of Boswelliapapyrifera (Del.) Hochst through leafless branch cuttings. Journal of Arid Environments 75:873-877. [ Links ]
Hartmann H.T., Kester D.E., Davies F.T. and Geneve R.L. 1997. Plant Propagation: Principles and Practices, 6th ed. Prentice Hall, New York. [ Links ]
Hernández-Apolinar M., Valverde T. and Purata S. 2006. Demography of Bursera glabrifolia, a tropical tree used for folk wood-crafting in Southern Mexico: an evaluation of its management plan. Forest Ecology and Management 223:139-151. [ Links ]
Hernández-Pérez E., González-Espinosa M., Trejo I. and Bonfil C. 2011. Distribución del género Bursera en el estado de Morelos, México y su relación con el clima. Revista Mexicana de Biodiversidad 82:964-976. [ Links ]
Hersch-Martínez P, Glass R., Fierro A.A. and Guerrero B.C. 2004. El linaloe, Bursera aloexylon (Schiede ex Schltdl) Engl. Programa actores sociales de la flora medicinal de México. Serie Patrimonio Vivo 6. Instituto Nacional de Antropología e Historia, Comisión Nacional para el Conocimiento y uso de la Biodiversidad, México, D.F [ Links ]
Hersch-Martínez P. and Glass R. 2006. Linaloe: Un Reto Aromático. Diversas Dimensiones de una Especie Mexicana, Bursera linanoe. Colección Científica Vol. 498. Serie Etnohistoria. Instituto Nacional de Antropología e Historia, México D.F. [ Links ]
Husen A. and Pal M. 2006. Variation in shoot anatomy and rooting behavior of stem cuttings in relation to age of donor plants in teak (Tectona grandis Linn. f.). New Forests 31:57-73. [ Links ]
Husen A. and Pal M. 2007a. Effect of branch position and auxin treatment on clonal propagation of Tectona grandis Linn. f. New Forests 34:223-233. [ Links ]
Husen A. and Pal M. 2007b. Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatments. New Forests 33:309-323. [ Links ]
Janzen D.H. 1988. Management of habitats fragments in a tropical dry forest: growth. Annals of the Missouri Botanical Garden 75:105-116. [ Links ]
Johnson M.B. 1992. The genus Bursera (Burseraceae) in Sonora, Mexico and Arizona, U. S. A. Desert Plants 10:126-143. [ Links ]
Kibbler H., Johnston M.E. and Williams R.R. 2004a. Adventitious root formation in Backhousia citriodora F. Muell: 1. Plant genotype, juvenility and characteristics of cuttings. Scientia Horticulturae 102:133-143. [ Links ]
Kibbler H., Johnston M.E. and Williams R.R. 2004b. Adventitious root formation in Backhousia citriodora F. Muell: 2. Seasonal influence of temperature, rainfall, flowering and auxins on the stock plant. Scientia Horticulturae 102:343-358. [ Links ]
Landis T.D., Tinus R.W. and Barnett J.P. 1999. The Container Tree Nursery Manual. Vol. 6. Seedling Propagation. Agriculture Handbook 674. USDA Forest Service. Washington, DC. [ Links ]
Leakey R.R.B. 2004. Physiology of vegetative reproduction. In: Burley J., Evans J. and Youngquist J.A. Eds. Encyclopedia of Forest Sciences, pp.1655-1668, Academic Press, London. [ Links ]
Lebrija-Trejos E., Bongers F., Pérez-García E.A. and Meave J.A. 2008. Successional change and resilience of a very dry tropical deciduous forest following shifting agriculture. Biotropica 40:422-431. [ Links ]
Leopold A.C. and Kriedemann P.E. 1975. Plant Growth and Development. 2nd ed. McGraw Hill, New York. [ Links ]
Linares E. and Bye R. 2008. El copal en México. Biodiversitas 78:8-11. [ Links ]
Maradiga F. S., Urban G. and Villerías S. 2000. Ecología de especies vegetales útiles del trópico de Guerrero. In: Monroy R., Colín H., and Boyas D.J.C. Eds. Los Sistemas Agroforestales de Latinoamérica y la Selva Baja Caducifolia en México, pp. 455-463, IICA-INIFAP. Universidad Autónoma del Estado de Morelos, Cuernavaca. [ Links ]
McVaugh R. and Rzedowski J. 1965. Synopsis of the genus Bursera L. in western Mexico, with notes on the material of Bursera collected by Sessé & Mociño. Kew Bulletin 18:317-382. [ Links ]
Mesén F., Newton A.C. and Leakey R.R.B. 1997. Vegetative propagation of Cordia alliodora (Ruiz & Pavon) oken: the effects of IBA concentration, propagation medium and cutting origin. Forest Ecology and Management 92:45-54. [ Links ]
Miles L., Newton A.C., DeFries R.S., Ravilious C., May I., Blyth S., Kapos V. and Gordon J.E. 2006. A global overview of the conservation status of tropical dry forests. Journal of Biogeography 33:491-505. [ Links ]
Murphy PG. and Lugo A.E. 1986. Ecology of tropical dry forest. Annual Review of Ecology and Systematics 17:66-88. [ Links ]
Negash L. 2002. Successful vegetative propagation techniques for the threatened African pencil cedar (Juniperus procera Hoechst. ex Endl.). Forest Ecology and Management 161:53-64. [ Links ]
Negash L. 2003. Vegetative propagation of the threatened African wild olive [Olea europaea L. subsp. causpidata (Wall. ex DC) Ciffieri]. New Forests 26:137-146. [ Links ]
Negash L. 2004. Stump sprouts as sources of cutting production for the vegetative propagation of the threatened African wild olive (Olea europaea L. subsp. cuspidata). South African Journal of Botany 70:24-30. [ Links ]
Nouaim R., Mangin G., Breuil M.C. and Chaussod R. 2002. The argan tree (Argania spinosa) in Morocco: propagation by seeds, cuttings and in-vitro techniques. Agroforestry Systems 54:71-81. [ Links ]
Ofori D. A., Newton A.C., Leakey R.R.B. and Grace J. 1996. Vegetative propagation of Milicia excelsa by leafy stem cuttings: effects of auxin concentration, leaf area and rooting medium. Forest Ecology and Management 84:39-48. [ Links ]
Purata S.E., Chibnik M., Brosi B.J. and López A.M. 2004. Figuras de madera de Bursera glabrifolia H. B. K. (Engl.) en Oaxaca, México. In: Alexiades M.N. and Shanley P Eds. Productos Forestales, Medios de Subsistencia y Conservación. Estudios de Caso sobre Sistemas de Manejo de Productos Forestales No Maderables, Volumen 3 - América Latina, pp. 415-437. CIFOR, Bogor Barat. [ Links ]
Puri S. and Verma R.C. 1996. Vegetative propagation of Dalbergia sissoo Roxb. using softwood and hardwood cuttings. Journal of Arid Environments 34:235-245. [ Links ]
Ray G. J. and Brown B.J. 1995. Restoring Caribbean dry forests: Evaluation of tree propagation techniques. Restoration Ecology 3:86-94. [ Links ]
Romero-Duque L.P.., Jaramillo V.J. and Pérez-Jiménez A. 2007. Structure and diversity of secondary tropical dry forests in Mexico, differing in their prior land-use history. Forest Ecology and Management 253:38-47. [ Links ]
Rzedowski J. and Kruse H. 1979. Algunas tendencias evolutivas en Bursera (Burseraceae). Taxon 28:103-116. [ Links ]
Rzedowski J., Medina L.R. and Calderón R.G. 2004. Las especies de Bursera (Burseraceae) en la cuenca superior del río Papaloapan (México). Acta Botanica Mexicana 66:23-151. [ Links ]
Sánchez-Azofeifa G.A. and Portillo-Quintero C. 2011. Extent and drivers of change of Neotropical seasonally dry tropical forests. In: Dirzo R., Young H.S., Mooney H.A. and Ceballos G. Eds. Seasonally Dry Tropical Forest. Ecology and Conservation, pp. 45-57, Island Press, Washington, DC. [ Links ]
Scott P.E. and Martin R.F. 1984. Avian consumers of Bursera, Ficus and Ehretia fruit in Yucatán. Biotropica 16:319-323. [ Links ]
Simons A.J. and Leakey R.R.B. 2004. Tree domestication in tropical agroforestry. Agroforestry Systems 61:167-181. [ Links ]
Swamy S.L., Puri S. and Kanwar K. 2002. Propagation of Robinia pseudoacacia Linn. and Grewia optiva Drummond from rooted stem cuttings. Agroforestry Systems 55:231-237. [ Links ]
Tchigio I. and Duguma B. 1998. Vegetative propagation of Calliandra calothyrsus (Meissner). Agroforestry Systems 40:275-281. [ Links ]
Tchoundjeu Z., Avana M.L., Leakey R.R.B., Simons A.J., Assah E., Duguma B. and Bell J. M. 2002. Vegetative propagation of Prunus africana: effect of rooting medium, auxin concentrations and leaf area. Agroforestry Systems 54:183-192. [ Links ]
Trejo I. and Dirzo R. 2000. Deforestation of seasonally dry tropical forests: a national and local analysis in Mexico. Biological Conservation 94:133-142. [ Links ]
Velázquez-Herrera J. 2011. Biología reproductiva de dos especies del género Bursera. Thesis, Facultad de Ciencias, Universidad Nacional Autónoma de México, México D. F. 68 pp. [ Links ]
Williams-Linera G., Alvarez-Aquino C., Hernández-Ascención E. and Toledo M. 2011. Early successional sites and the recovery of vegetation structure and tree species of the tropical dry forest in Veracruz, Mexico. New Forests 42:131-148. [ Links ]
Zahawi R.A. 2005. Establishment and growth of living fence species: an overlooked tool for the restoration of degraded areas in the tropics. Restoration Ecology 13:92-102. [ Links ]
Zahawi R.A. and Holl K.D. 2009. Comparing the performance of tree stakes and seedlings to restore abandoned tropical pastures. Restoration Ecology 17:854-864. [ Links ]














