Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Botanical Sciences
versão On-line ISSN 2007-4476versão impressa ISSN 2007-4298
Bot. sci vol.90 no.3 México Set. 2012
Ecology
Edaphic and seasonal heterogeneity of seed banks in agricultural fields of a tropical dry forest region in southern Mexico
Heterogeneidad edáfica y estacional de los bancos de semillas en campos agrícolas de una región de bosque tropical seco del sur de México
Jorge A. Meave1, Claudia Flores-Rodríguez, Eduardo A. Pérez-García and Marco Antonio Romero-Romero
Departamento de Ecología y Recursos Naturales, Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad Universitaria, México 04510, Distrito Federal, Mexico.
1Corresponding author: jorge.meave@ciencias.unam.mx
Received: September 29, 2011
Accepted: March 14, 2012
Abstract
The slash-and-burn agriculture practiced across tropical dry regions results in the elimination of native vegetation. Upon field abandonment, the seed bank becomes a potentially important mechanism of natural regeneration at early successional stages. Soil properties and climate seasonality may affect seed bank characteristics, thus we analyzed the effects of these two factors on seed bank density and composition in agricultural fields of a seasonally dry tropical region of southern Mexico. Soil cores were collected for the rainy and the dry seasons in order to assess changes occurring in the seed bank from the time of harvest to the moment when succession could potentially start (the next rainy season). The 12 studied fields comprised three different soil types recognized by local inhabitants: sandy and stony, silty, and clayey soils, locally known as cascajo, black soil, and red soil, respectively. At each field 20 soil cores (8 cm diameter, 4.5 cm depth) were collected and mixed to form four pooled samples, which were placed in a greenhouse to induce germination. A total of 4,422 seedlings (2,291 seeds m-2) representing 40 species were recorded. The most abundant species were, in decreasing order, Melanthera nivea, Rhynchelytrum repens, Waltheria indica, Amaranthus scariosus, Digitaria bicornis, and Cenchrus pilosus. Herbs were the prevailing growth form (> 80% of total richness). No clear pattern was observed in the seed bank related to soil type; however, seed bank characteristics tended to be associated with the time of use of the agricultural fields, a variable that was not controlled in the study. Seed bank species richness was significantly larger in the dry season, and although seed density showed a similar trend, it was not significant. The studied seed banks contain no elements of the regional primary tropical dry forest, which suggests that seed banks in deforested areas cannot guarantee their maintenance beyond forested areas.
Key words: seed bank seasonal variation, seed germination, soil heterogeneity, tropical forest regeneration, weed ecology.
Resumen
La agricultura de roza, tumba y quema practicada en regiones tropicales secas provoca la eliminación de la vegetación original. Cuando los campos agrícolas son abandonados, el banco de semillas puede ser un mecanismo importante de regeneración natural en etapas tempranas. Las propiedades del suelo y la estacionalidad climática pueden afectar las características del banco de semillas. En este estudio analizamos los efectos de estos dos factores sobre la densidad y la composición del banco de semillas de suelos agrícolas de una región de bosque tropical estacionalmente seco del sur de México. Se obtuvieron núcleos de suelo de la temporada de lluvias y de la seca para evaluar los cambios en el banco de semillas desde la cosecha hasta el momento en el que la sucesión podría comenzar (la siguiente temporada lluviosa). Los 12 sitios de estudio abarcaron tres tipos diferentes de suelos reconocidos por los habitantes locales: arenosos y pedregosos, con alto contenido de limo, y arcillosos, conocidos localmente como cascajo, suelo negro y suelo rojo, respectivamente. En cada campo se recolectaron 20 núcleos de suelo (diámetro 8 cm, profundidad 4.5 cm), los cuales se mezclaron para obtener cuatro muestras compuestas por sitio. Éstas fueron colocadas en un invernadero para promover la germinación de las semillas. Registramos un total de 4,422 plántulas (2,291 semillas m-2) representadas por 40 especies, siendo Melanthera nivea, Rhynchelytrum repens, Waltheria indica, Amaranthus scariosus, Digitaria bicornis y Cenchrus pilosus las más abundantes en este orden. Las hierbas fueron la forma de crecimiento predominante (> 80% de la riqueza total). No hubo un patrón claro de variación en el banco de semillas relacionado con el tipo de suelo. Sin embargo, las características del banco parecen estar asociadas con el tiempo de uso del campo, variable que no fue controlada en este estudio. La riqueza de especies fue significativamente mayor durante la época seca, y aunque la densidad de semillas mostró una tendencia semejante, ésta no fue significativa. Los bancos de semillas estudiados no contienen elementos del bosque tropical seco primario de la región, lo que sugiere que los bancos de semillas de áreas deforestadas no pueden asegurar el mantenimiento de estos sistemas fuera de las áreas con bosque.
Palabras clave: ecología de malezas, germinación de semillas, heterogeneidad edáfica, regeneración del bosque tropical, variación estacional del banco de semillas.
Tropical dry forests (TDFs) have been severely modified or destroyed worldwide owing to human activities (Janzen, 1988; Lerdau et al., 1991; Fajardo et al., 2005; Sánchez-Azofeifa et al., 2005). In Mexico, unchecked human population growth and the ever-increasing need to produce food have resulted in a constant opening of agricultural fields and pastures in TDF regions in the last decades (Challenger, 1998; Trejo and Dirzo, 2000; Burgos and Maass, 2004; Maass et al., 2010). Several factors have been proposed to contribute to the speedy conversion of TDF into productive systems, including the relative ease of clearing the short-statured forest, and the presence of a suite of less aggressive weeds compared to those from tropical humid regions (Murphy and Lugo, 1986). Agricultural fields in TDF regions are often abandoned after one or a few crop cycles based on slash-and-burn practices, mostly due to fertility loss and soil erosion (Ewel et al., 1981; Maass et al., 1988; Jaramillo et al., 2010), in addition to other less-well understood factors such as the increase in weed density.
Upon abandonment of agricultural fields a natural process of vegetation recovery takes place; however, several factors may hinder or delay the course of succession (Uhl et al., 1988; Chapman and Chapman, 1999; Holl, 1999; Wijdeven and Kuzee, 2000; Meli, 2003). Successional routes of recovering plant communities are largely dependent on a few regeneration strategies displayed by plants (Grime, 1979; Oliver, 1980), which in turn show a certain dependence on previous land uses (Romero-Duque et al., 2007). Common regeneration mechanisms in abandoned fields occurring in different ecosystems are: (1) re-sprouting from stumps and roots that survived the agricultural process (Miller and Kauffman, 1998; Vieira and Scariot, 2006), (2) germination from newly dispersed propagules into the site (Ramírez-Marcial et al., 1992; Ekeleme et al., 2000; Kennard et al., 2002), and (3) germination of seeds that remained in situ, deposited in the soil seed bank (Ewel et al., 1981; Quintana-Ascencio et al., 1996; Hyatt and Casper, 2000; Kennard et al., 2002; Luzuriaga et al., 2005).
Germination of seeds from the soil seed bank in systems repeatedly subjected to intense fires may be an important driver of vegetation recovery (Kennard et al., 2002), particularly in TDF areas widely affected by slash-and-burn agriculture (Skoglund, 1992), because in those areas stumps and roots tend to disappear completely after such practices. However, there is also evidence showing limited establishment from seeds at early successional stages due to a shortage of propagules (Uhl et al., 1981). Similarly, seed banks appear to contribute more to the establishment of herbaceous than of woody species (Rico-Gray and García-Franco, 1992; Miller, 1999; Lemenih and Teketay, 2006), particularly when the influx of seeds of forest species is limited due to the lack of nearby forest fragments in the landscape (Holl, 1999; Wijdeven and Kuzee, 2000; Cubiña and Aide, 2001).
Though there is much less information on seed bank characteristics in TDF regions (Rico-Gray and García-Franco, 1992; Miller, 1999; Khurana and Singh, 2001) than for the humid tropics (e.g. Kellman, 1974; Uhl et al., 1981; Quintana-Ascencio et al., 1996; Guevara et al., 2005), the combined evidence from all tropical regions allows the identification of the gamut of factors potentially affecting tropical seed bank characteristics. These factors comprise, inter alia, time of use of the agricultural fields (Kellman, 1974; Ewel et al., 1981; Pickett and McDonnell, 1989; Burgos and Maass, 2004; Guevara et al., 2005), distance to and the type of surrounding vegetation (Quintana-Ascencio et al., 1996; Dalling and Hubbell, 2002; Dalling et al., 2002; Guevara et al., 2005), agricultural practices (Ewel et al., 1981; Miller, 1999; Kennard et al., 2002; Lemenih and Teketay, 2006), and soil conditions (Uhl and Clark, 1983; Cavers and Benoit, 1989).
Regarding the influence of soil attributes on seed bank density and composition, different components of the edaphic variation in a region can be clearly related in complex ways to seed bank composition and dynamics (e.g. Russell-Smith and Lucas, 2009); for example, Kellman (1974) found a negative effect of acidic pH on species richness. Perhaps the most important soil physical trait affecting seed banks is soil texture, as different textures can aid or restrict the entrance of seeds into the soil, and later they can trigger differential germination responses because of differences in water retention, the probability of seeds to become scarified under a given soil particle size distribution, and the differences in soil porosity and the consequent differential gas exchange and light penetration (Paatela and Erviö, 1971; Vincent and Cavers, 1978; Pareja and Staniforth, 1985; Colosi et al., 1988; Wild, 1993; Baskin and Baskin, 1998).
A further relevant factor for the study of soil seed banks in TDF regions is its temporal behavior associated to rainfall seasonality. Seasonal precipitation causes distinct pulses of fruit maturation, seed production and germination, and seedling establishment throughout the year (McLaren and McDonald, 2005); for example, previous studies have suggested the existence of a post-harvest increase in the number of seeds (and species) in the soil seed banks of these environments due to increased propagule maturation and dispersal during the dry season (Garwood, 1983; Dalling et al., 1998; Pérez and Santiago, 2001).
The goals of this study were twofold. First we analyzed the effect of soil type on the variability of seed bank size and species composition in a TDF region of southern Mexico. Second, we evaluated seasonal changes in seed bank characteristics from the time of crop harvest to the end of the following dry season, when secondary succession could potentially start upon field abandonment. Our ultimate aim was to assess the possible role of the seed bank in the regeneration of this TDF. We hypothesized that the different soils recognized by local farmers would be associated with differences in seed bank characteristics. Also, given the variations in the activity of soil biota depending on water availability in the system, we expected the seed bank to be richer and denser at the end of the dry season, although previous studies have reported contradictory results on this issue (Grombone-Guaratini and Rodrigues, 2002; Martins and Engel, 2007).
Study region
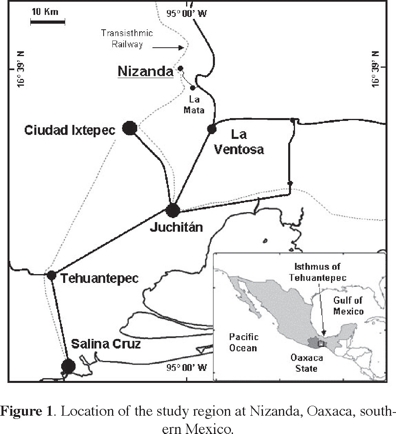
The fields where the soil seed banks were studied are located in the vicinity of Nizanda (16° 39' N, 95º 00' W), a small village found in the southern portion of the Isthmus of Tehuantepec, Oaxaca State, southern Mexico (Figure 1). Elevation in the area ranges from 100 to 750 m a.s.l., but most agricultural fields lie between 100 and 300 m. The main bedrock in the area where these fields are located is a matrix of metamorphic rocks, mainly siliciclastic phyllite (Pérez-Gutiérrez et al., 2009). Climate is typical of the Pacific watershed lowlands in southern Mexico, i.e., warm sub-humid, with summer to mid-autumn (June–October) rains. On average, total annual precipitation is ca. 900 mm, and mean annual temperature is 26 °C. After the relatively short rainy season, precipitation is almost non-existent, except for some isolated rain events caused by incoming cold air masses (nortes) from the Gulf of Mexico during fall and winter.
Agroecosystems still cover a relatively minor proportion of the study region (Gallardo-Cruz et al., 2010). Except for those fields located on deeper soils in the floodplains of the intermittent stream known as Río Verde, the large majority of agricultural fields are relatively small, usually < 0.5 ha (Lebrija-Trejos et al., 2008). The main crop in Nizanda is maize or corn, which is usually grown in mixture with squash, but there is an increasing trend to produce cash crops such as sesame and sorghum. As in other TDF regions of Mexico, Central America, and elsewhere, the first step in the agricultural cycle is land preparation, which is done through the slash-and-burn system. Afterward, soils may be plowed but occasionally seeds are planted with a coa (traditional planting stick) without previous plowing. Harvest takes place in the late rainy season, usually in October. When a field is not abandoned, farmers prepare the land for one more agricultural cycle at the end of the following dry season.
Materials and methods
Site selection and soil description. The farmers of Nizanda distinguish three soil types: cascajo (a term roughly equivalent to pebbles), black soil, and red soil. Previous studies have demonstrated the usefulness and accuracy of traditional soil classifications developed by local peoples (Barrera-Bassols and Zinck, 2003; Cervantes-Gutiérrez et al., 2005). Cascajo refers to a relatively shallow, sandy and stony soil that is common on moderately-sloped hillsides. Black soils are silty and considerably less stony than cascajo, and often occur in flat land. Stoniness is also low in red soils, but these are more clayey, and are mostly located on flat terrain as well. With the assistance of local farmers we chose a group of 12 fields, with four fields representing each soil type. All selected fields were being used for food production when the study started, and they were harvested just before the first soil core collection.
For each site we provided a broad description of surface soil characteristics (to 40 cm depth). Soil description was done following Siebe et al. (1996) and included soil texture (assessed through the 'tact test' of the fine soil fraction [< 2 mm] for determining malleability, consistency and granularity), stoniness (percent volume occupied by pebbles and stones), color (according to The Munsell® Book of Color, Glossy Collection), pH (indicator paper), pF (soil suction, a moisture-retaining characteristic of the soil, assessed through direct observation by tact and color change when adding water), soil structure (aggregate shape and size), aggregate stability (degree of disintegration of aggregates when placed in water), and bulk density (indirectly assessed through the effort required to insert a knife in the upper soil horizon when dry). For each agricultural field, time of use was obtained by interviewing the owner.
Seed bank sampling. Soil samples were collected in two seasons. The first sampling was done in November 2005, just after the harvest and at the end of the rainy (wet) season. The second set of soil samples was collected in March 2006, at the peak of the following dry season, but before the fields were burned as part of site preparation for the subsequent agricultural cycle. The first samples were assumed to represent seed bank conditions just after disturbance (agriculture) cessation, whereas dry season samples were assumed to exemplify seed bank features at the time when succession would start, should the field be abandoned. Hereafter these two sets will be referred to as the wet season and the dry season samples, respectively.
At each site (i.e. agricultural field), five soil cores were collected at 5 m intervals along each of four 20 m long compass lines set up centrally in the field and 7 m apart from each other. Where plough furrows were still visible, compass lines were set up perpendicularly to them and sample collection alternated between the crests and bottoms of the furrows. The decision to take 20 soil cores per site was based on the benefits derived from collecting more samples by site rather than larger but fewer samples (Benoit et al., 1989; Butler and Chazdon, 1998). Given the well-established decreasing pattern of seed density with increasing depth in the soil (Dalling et al., 1998; Luzuriaga et al., 2005), cores were collected from the surface soil (8 cm diameter, 4.5 cm deep; area = 50.27 cm2, volume = 226.19 cm3).
The five soil cores collected along each line were mixed thoroughly to produce a single pooled sample per line. Pooled samples were placed in paper bags and kept in cardboard boxes for transportation to Mexico City. Samples were stored for some time (< 4 months) in dark, cool conditions due to limited greenhouse space availability.
Seed bank composition. Owing to the difficulty to determine directly the taxonomic identities of the seeds stored in the seed bank, we opted for the commonly used germination method to examine seed bank composition (Poiani and Carter-Johnson, 1988; Gross, 1990). After sieving to discard stones and organic remains, a fraction representing 80% of the pooled samples was placed in 26 × 52 cm plastic trays previously filled with a 2.5 cm deep layer of agrolite/vermiculite (2:1) mixture; the total soil surface represented in each tray was 804.32 cm2, and correspondingly for each soil type total surface was 3,217.3 cm2. These figures were used to calculate seed density per meter squared.
The trays were placed in the greenhouse using a random block design; four blocks were established, each containing an equal number of samples for each site and soil type. The trays were inspected for seed germination for 18 weeks, initially at three-day intervals and weekly thereafter. Individual germinated seeds were marked with color-coded toothpicks and bijoux to prevent double tallying. The soil was kept moist by spraying water as frequently as needed. Air temperature in the greenhouse ranged between 11 °C and 43 °C, with a mean temperature of 22.4 °C.
Seedlings emerging from germinated seeds were allowed to grow for a few days until it was safe to transfer them to individual containers to grow in a greenhouse. Taxonomic determination was achieved by comparison with our herbarium specimens from the study region (Pérez-García et al., 2001; 2010) or with the aid of specialist taxonomists at our institution.
Data analysis. Two-way ANOVAS were performed with Statistica 8 (StatSoft Inc., 2007) to assess the effects of soil type and season on seed density and species richness; data were square-root transformed to meet normality assumptions (Zar, 1999).
Changes in species composition between seasons for each field were assessed by calculating the floristic similarity between them using the Jaccard coefficient (Kent and Coker, 1994). In addition, two hierarchical classifications of the studied fields were performed, one based on species incidence (presence/absence) data, and the other on species abundances (proportions of seeds by species) stored in the seed banks. In both cases the clustering was done with Ward's (minimum variance) method and using Euclidean distances as dissimilarity measure (Kent and Coker, 1994). This analysis was also performed with Statistica.
Results
Between-field comparison of soil traits and use history. The three soil types were similar regarding pH (range: 6-7), soil suction (pF range: 4-5), bulk density (mostly high but medium at three sites), and aggregate shape and stability (moderately unstable to very unstable clods or fragments), but they showed marked differences in texture and stoniness (Table 1). Cascajo soils were mainly sandy loam and had angular pebbles (63-200 mm) accounting for a much larger proportion (30-50%) of soil volume than in the other two soil types. Black soils had a silt loam texture predominantly and contained small to large pebbles (2-63 mm) that occupied between 2-5% of soil volume. Red soils were mostly clay loam and contained large pebbles (20-63 mm) representing between 1-10% of their soil volume.
In addition to soil properties, we also recorded between-site differences in the land-use history and the vegetation surrounding the fields (Table 1). Sites with cascajo soils were always located on hillsides, unlike those on the other soil types, which were always located on flat terrain. Regarding other characteristics, there were no clear trends in time of use and surrounding vegetation for the fields located on each soil type. Time of use had a bimodal frequency distribution, as the set of fields had been either cultivated for just one year or for periods ranging from 10 to 65 yr. Similarly, surrounding vegetation included various combinations of secondary vegetation, maize fields and in a few cases (excepting black soils), mature forest, without any clear trend associated with soil type.
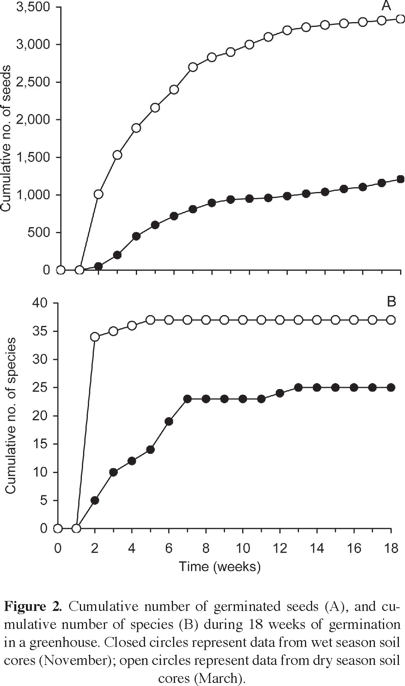
Overall characterization of the seed banks. Cumulative curves of numbers of germinated seedlings and species in the soil cores for the wet and the dry season are given separately in figure 2. In general, these curves showed clear stabilizing trends, particularly those pertaining to number of species. The only exception was the cumulative curve for germinated seeds in the wet season, which continued to increase even after four months. Species richness did not show any increase beyond 13 weeks in the case of wet season cores, and beyond five weeks in those from the dry season.
A grand total of 4,422 seeds germinated in the greenhouse; seedlings represented 40 morphospecies distributed in 18 families (Appendix 1). Overall mean (± S.E.) seed bank density in the fields was 2,291 ± 639 seeds m2. Thirty-three morphospecies were determined to species level, two to genus level, and one to family level only; the taxonomic adscription of four seed morphs remained unknown at all levels. Poaceae was the most speciose family (8 species), followed by Fabaceae (4). The seven families having two or more species accounted for over 62.5% of total richness, whereas the remaining 11 families, each represented by a single species, only accounted for 27.5% (Figure 3).

The most abundant species in the seed bank, regardless of soil type or season and in decreasing order, were Melanthera nivea, Rhynchelytrum repens, Waltheria indica, Amaranthus scariosus, Digitaria bicornis, and Cenchrus pilosus. These six species accounted for 63% of all germinated seeds. Species with the highest frequencies across sites were R. repens (10 sites), W. indica (10) and Caryophyllaceae sp. (9); no single species was recorded at all 12 sites even after pooling the information for the two seasons.
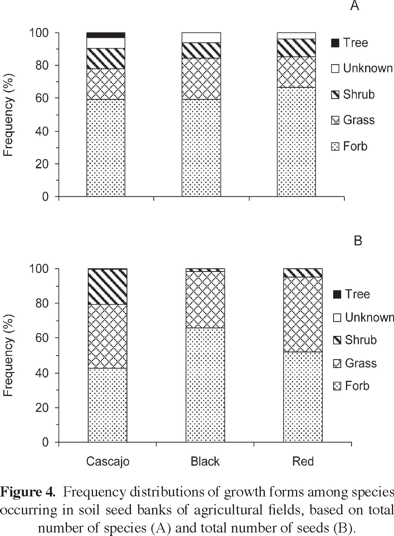
Growth form distribution. The relative proportions of growth forms in the seed banks of the three soils are shown on figure 4, based both on number of species (Figure 4A) and number of seeds (Figure 4B). According to the number of species, herbs (forbs and grasses together) were the prevailing life form; the small remaining fractions were mostly shrubs or species with unknown life form, and only cascajo soils contained one tree species in the seed bank. The pattern obtained from the density-based analysis was similar, except that the categories corresponding to unknown life forms and trees virtually disappeared due to a very low representation. Moreover, the proportion of shrubs in cascajo soils increased notably, although this increase was largely due to the very abundant Waltheria indica. In addition, the proportions of grasses in all soil types increased.
Effects of soil type and season on seed bank density. The analysis of variance showed that neither soil type nor season (or their interaction) had significant effects on seed bank density (Table 2). Nonetheless, the differences in the means calculated for this variable were noticeable (Appendix 2). Mean seed densities in the three soil types tended to be higher in the dry season (Figure 5A). Also, mean seed density tended to be higher in cascajo (Figure 5B), and this variable was almost three-fold larger in the dry season than in the wet season for all soil types combined (Figure 5C).
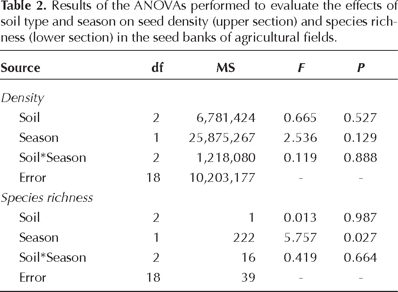
Effects of soil type and season on seed bank diversity. Total species richness in the seed bank varied only slightly in response to soil type (32 species in cascajo, 31 in black soils, and 27 in red soils). Mean richness was similar among the three soil types (Figure 5E). Nonetheless, there was a large difference in total richness between season (wet season, 25 species; dry season, 37 species; Appendix 1), and mean species richness differed significantly between seasons (Table 2). Mean species richness in the soil seed bank of the dry season almost doubled the value observed for the wet season (Figure 5F), and this between-season difference was maintained when each soil type was analyzed separately (Figure 5D). There was no significant effect of the soil × season interaction (Table 2). Moreover, when considering species composition regardless of species abundances, relatively low similarities between the samples of the dry and the wet seasons for each field were observed (mean Jaccard similarity index for each field by soil type: 27.5%, 16.3% and 34.3% for cascajo, black and red soils, respectively).
Despite the lack of between-soil differences in species richness and the modest total richness observed in the studied seed banks, there were some remarkable between-soil type differences in the identities of the most abundant species (Table 3). In cascajo soils Rhynchelytrum repens was the most abundant species, followed closely by Melanthera nivea and Waltheria indica (combined relative abundance: 61.1%). In turn, the most abundant species in black soils was Amaranthus scariosus, followed by Trianthema portulacastrum, M. nivea, and Panicum fasciculatum (combined relatively abundance: 64.7%). Finally, 65.5% of the seedlings recorded in red soils were accounted for Cenchrus pilosus (the most abundant species), M. nivea, Digitaria bicornis, and Euphorbia heterophylla.
Classification analysis. The dendrograms resulting from the classifications of the 12 fields based respectively on species incidence and abundance data in their pooled seed banks from the wet and the dry season are shown in figure 6. In the incidence-based classification, two well-defined and equal-sized groups were distinguished at a linkage distance of 5 (Figure 6A). One group included sites R2, R1, C4, B3, B1, and C3, while the other included sites R3, B4, R4, C2, B2 and C1.
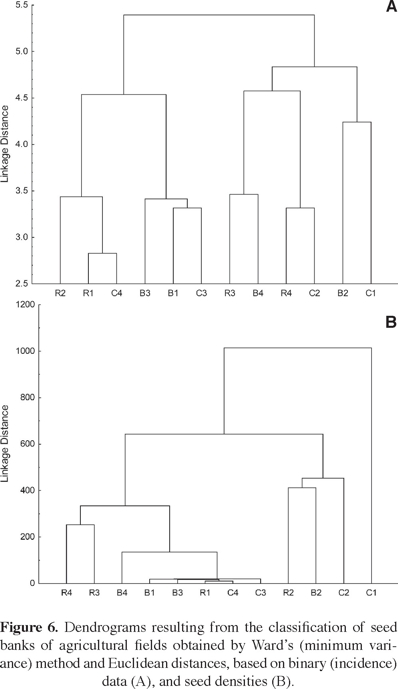
The abundance-based classification also formed two groups clearly distinguished at a cutoff distance of 500, with site C1 remaining as an unclassified outlier (Figure 6B). As in the previous case, this classification did not show any clustering pattern that could be related to soil type. Moreover, the strong dominance of a few very abundant species led to a dendrogram configuration different from that based on species incidence data: the first group (sites R4, R3, B4, B1, B3, R1, C4 and C3) was more numerous and homogenous than the second one (sites R2, B2 and C2).
Discussion
Seed bank size and composition. Both the total density of the seed bank in Nizanda (2,291 seeds m-2) and the number of species stored in it (40 species) have an intermediate position among figures reported for other tropical dry regions. Lemenih and Teketay (2006) found 1,425 seeds m-2 belonging to 66 species in the seed bank of agricultural soils in an Afromontane dry forest region in Ethiopia. In less distant tropical dry forest locations within Mexico, Rico-Gray and García-Franco (1992) reported 1,815 seeds m2 representing 29 species in Yucatán, whereas Miller (1999) found a higher density (3,502 seeds m-2) but a less rich composition (13 species) in Jalisco.
Among the most abundant species in the seed banks of the agricultural fields of Nizanda are Melanthera nivea (Asteraceae), the exotic Rhynchelytrum repens (Poaceae), and Waltheria indica (Sterculiaceae). These species, widely recognized weeds in the Mexican flora (Villaseñor and Espinosa-García, 2004), together represented 40% of all germinated seeds recovered from the soils. An overabundance of seeds of weedy species in seed banks of crop fields may modify the flora of recovering forests in the long-term (Quintana-Ascencio et al., 1996), considering their strong competitive abilities and rapidly growing populations (Zimdahl, 1999). However, it has also been suggested that they may have positive effects on early successional communities established in abandoned fields by providing favorable conditions for the establishment of later successional species (Uhl et al., 1981), particularly in the harsh near-ground micro-environments typical of early successional stages in tropical dry forests (Lebrija-Trejos et al., 2010, 2011).
In a study on forest succession conducted in our study region at sites with cascajo soils, Lebrija-Trejos et al. (2008) reported that Waltheria indica was the dominant species in the youngest fallows (relative abundance of 67% in a recently abandoned field, and 19% in a 1-yr old fallow). Thus, at least for this species, our study provides evidence that the initial composition and structure during the successional process of Nizanda is strongly influenced by its presence in the seed bank, as has been suggested for other sites (Garwood, 1989; Marod et al., 2002).
Growth forms. In TDF regions, herbs are often the most common growth form in seed banks of agricultural fields, accounting for as much as 90% of all seeds (Rico-Gray and García-Franco, 1992; Lemenih and Teketay, 2006). This fraction is slightly larger than those found in this study (77.5% of all species; 88.2% of all seeds). Dominance of herbs in seed banks of agricultural fields may be caused by several factors; for example, the small size of seeds of herbaceous species makes it easier for them to penetrate the soil (Zimdahl, 1999; Khurana and Singh, 2001). Also, the short reproductive cycles (sometimes even shorter than annual) and prompt seedling emergence facilitate their simultaneous establishment with the growing crop plants or shortly after they have been harvested when fields are left to rest. Newly-established populations of herbs in agricultural fields may trigger a positive feedback loop, as they will start producing large amount of seed shortly after their establishment (Uhl et al., 1982; Cavers and Benoit, 1989). At sites that were burned for a long time some seeds remain as long as they can tolerate high temperatures, and in some cases fire promotes their germination (Rico-Gray and García-Franco, 1992; Khurana and Singh, 2001). The majority of herbaceous species in the seed bank of Nizanda were forbs (58%), whereas grasses accounted only for 20% of the total. Grasses and grass-like species vary greatly in their representation in seed banks of tropical regions, with values ranging between 9 and 96% (Rico-Gray and García-Franco, 1992; Miller, 1999). At least in some regions it is suspected that cattle used for the agricultural tasks may be an efficient mechanism of bringing seeds into the fields (Uhl et al., 1988).
As is often the case, woody species had an extremely poor representation in the seed bank of Nizanda (five species; four shrubs, and one tree). This number is similar to the four woody species reported by Rico-Gray and García-Franco (1992), and the six species reported by Lemenih and Teketay (2006). Interestingly, the only tree species found in the seed bank of Nizanda, Acacia cochliacantha, is a common element in early successional stands in the region (Lebrija-Trejos et al., 2008); however, this is by no means the dominant species either in those fallows or in the mature forest. In the fallows, the dominant trees are Mimosa acantholoba var. eurycarpa and M. tenuifolia, but not a single seed of these species was found in the bank. The scarcity of mature forest tree and shrub species in the seed banks of agricultural fields suggests that their propagules face strong dispersal limitations; moreover, the few seeds that successfully arrive at the agricultural fields may be vulnerable to animal predation, pathogen attack, desiccation, or damage by fire (Quintana-Ascencio et al., 1996; Dalling et al., 1998; Lemenih and Teketay, 2006).
Seed bank heterogeneity. It has long been suggested that soil characteristics like texture and aggregate structure can affect seed bank dynamics in agricultural fields (Brenchley and Warington, 1930; Paatela and Erviö, 1971; Pareja and Staniforth, 1985; Colosi et al., 1988). The most important difference between the three soil types compared in this study was related to texture and stoniness. In spite of these differences, we failed to observe clear patterns in seed bank density and overall floristic structure that could be related to soil type. This was particularly clear from the results of the classification analysis. Similarly, the ANOVAs used to compare seed bank richness and density between soil types did not show any significant differences, despite a strong tendency of sites with cascajo soils to have higher densities (mean densities in black and red soils were one-half and two-thirds, respectively, in comparison with mean density in cascajo soils). Although in the case of seed density the lack of significant differences may be at least partially accounted for by the enormous variability observed for this trait within each soil type, this is certainly not the case for species richness.
The results of this study indicate that edaphic-related soil differences among sites do not play a key role in determining seed bank densities at the start of successional processes in Nizanda. However, our results also showed that this interpretation does not necessarily apply to the case of species dominance and growth-form spectra. On the one hand, the most abundant species in the seed banks were different for each soil type (Table 3); on the other, cascajo soils had a slightly larger proportion of seeds of shrubby species, particularly of Waltheria indica, one of the dominant species of early successional stages at sites with cascajo soil in the region (Lebrija-Trejos et al., 2008). Therefore, future studies on secondary succession that focus on sites located on the other soil types could prove the existence of early differences in the vegetation recovery process. Nonetheless, it is uncertain whether these differences would persist into late successional stages, given the apparent convergence towards a few dominant pioneer tree species in most of the area covered by the secondary vegetation across the region.
While analyzing the data it became evident that an originally unforeseen factor could potentially play a role in driving some seed bank traits. This evidence came from a closer inspection of the groups produced by the classification of agricultural fields based both on qualitative and quantitative data. In both cases the resulting groups were heterogeneous regarding soil type. However, there was an apparent separation between fields that had been used during short periods of time and fields with a longer history of use. We thus performed a non-parametric Spearman correlation analysis between time of use and two seed bank characteristics, namely seed density and species richness. Time of use was significantly correlated with total seed density (Figure 7A), and with dry-season mean seed density (Figure 7C), but it was not so with seed bank species richness of any data set (Figure 7D, E, F). The observed significant correlations could be associated with the effects of the continuous slash-and-burn agriculture practiced on these fields (Miller, 1999; Kennard et al., 2002; Lemenih and Teketay, 2006). As time goes by, seed bank content may increase by the accumulation of seeds of early successional or weedy species, whose life cycles are finely adapted to the growing season for crops (Uhl et al., 1982; Cavers and Benoit, 1989). However, it must be acknowledged that the relationships shown on figure 7 also suggest the existence of other unknown factors capable of producing high seed bank densities even in fields that were used for a few years.
Seasonal changes in the seed bank. For some seasonally dry tropical systems larger seed bank densities have been reported for the dry season (Garwood, 1983; Dalling et al., 1997; Pérez and Santiago, 2001). The between-season difference in this seed bank trait has been interpreted as a result of the permanence of some seeds with seasonal dormancy during the drought, given that several species have their propagules dispersed in this time of the year, and because of the exogenous dormancy common in them (Garwood, 1989). In this study we also observed several changes in the seed bank size and composition between the wet and the dry seasons. Particularly important was the significant increase in seed bank richness in all 12 sites from the wet to the dry season, along with a (non-significant) trend towards a higher seed density in the dry season. That is, the seed bank grew and became richer from the time of harvest to the moment when seeds in the seed bank could have germinated.
Our results show that the between-season changes in the studied seed banks were not of the same magnitude at all sites. An examination of the changes occurring in the seed banks of the different sites between the two seasons also indicates that their traits did not became homogeneous with time between harvest and the peak of the dry season, suggesting strong idiosyncrasies in the studied seed banks. Between-season differences in seed bank traits can also be interpreted as an indication of the transient character of the seed banks in the agricultural fields at Nizanda (sensu Garwood, 1989), but this character may be species-specific rather than a community trait.
Potential role of seed bank in TDF regeneration. The combined evidence discussed in the previous sections leaves some important unresolved questions regarding the role of the seed bank in the regeneration of the TDF in our study region.
For one, it is clear that the seed bank in recently abandoned fields of Nizanda hosts virtually no woody species; thus the soil seed bank seems to play a very minor role in promoting TDF regeneration. Although this result contrasts with the findings of other studies that have analyzed seed banks of tropical regions (e.g. Grombone-Guaratini and Rodrigues, 2002), it resembles those from studies performed in successional TDF vegetation (Mena-Gallardo, 2009; Maza-Villalobos et al., 2011).
A strong motivation to conduct this study was the observation that at Nizanda very young fallows are dominated by an array of herbaceous, shrubby, and suffruticose plants for a short time (ca. 1-2 yr), after which trees belonging to a few spiny species of the Mimosaceae family take over the community, with an almost complete absence of mature forest trees in the forest canopy for almost 30 yr (Lebrija-Trejos et al., 2008). To our surprise, not only were these Mimosaceae trees not represented at all in the studied seed banks, but other potential pioneer tree species occurring in the region did not have seed banks either, including Cnidoscolus megacanthus Breckon, Cochlospermum vitifolium (Willd.) Spreng., Gliricidia sepium (Jacq.) Kunth, Guazuma ulmifolia Lam., and Heliocarpus pallidus Rose. We suggest that future studies should examine the possibility that the establishment of all these species in abandoned crop fields depends on propagule dispersal.
Conclusions
The bulk of seeds contained in the soil banks of agricultural fields at Nizanda correspond to herbaceous and shrub species. The most abundant species in the seed bank are Melanthera nivea, Rhynchelytrum repens, and Waltheria indica, which are all well known because of their weedy behavior. Contrary to our expectations, this study only provided evidence of a weak influence of soil type on seed bank characteristics in these environments; however, time of use of the fields emerged as a potential factor capable of affecting seed bank features, given the increases in richness and seed density with the increasing time of use of the agricultural field. Future research should pay more attention to this explanatory variable, as it was not adequately controlled for in this study.
Our results clearly show that the studied seed banks contain virtually no elements of the primary tropical dry forest or of other plant communities of the region. This emphasizes the need to preserve some tracts of primary forest, as the seed banks of deforested areas do not seem to be capable of ensuring their maintenance beyond forested areas through a passive restoration process.
Acknowledgments
We are grateful to Héctor Benavides and Francisco Camacho for the technical assistance and the opportunity to use a greenhouse at INIFAP (National Institute for Forest, Agriculture and Livestock Research). Field- and lab work was kindly assisted by the Reyes Manuel family, Oswaldo Núñez, Alejandra Mena, Gerardo Cervantes, Yuriana Martínez, as well as the owners of the studied fields. Beatriz González and Ramiro Valencia helped with seedling taxonomic determination, and Edwin Lebrija provided theoretical guidance. The comments and criticism of two anonymous reviewers on an earlier version helped improve this article. Thanks to Trudy Kavanagh for carefully editing this manuscript. This research was funded by PAPIIT-UNAM (IN221503-3 and IN216007-3) and CONACyT (grant no. 128136).
References
Barrera-Bassols N. and Zinck J.A. 2003. Ethnopedology: a worldwide view on the soil knowledge of local people. Geoderma 111:171-195. [ Links ]
Baskin C.C. and Baskin J.M. 1998. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. Academic Press, San Diego. [ Links ]
Benoit D.L., Kenkel N.C. and Cavers P.B. 1989. Factors influencing the precision of soil seed bank estimates. Canadian Journal of Botany 67:2833-2840. [ Links ]
Brenchley W.E. and Warington K. 1930. The weed seed population of arable soil: I. Numerical estimation of viable seeds and observations on their natural dormancy. Journal of Ecology 18:235-272. [ Links ]
Burgos A. and Maass J.M. 2004. Vegetation change associated with land-use change in tropical dry forest areas of Western Mexico. Agriculture, Ecosystems & Environment 104:475-481. [ Links ]
Butler B.J. and Chazdon R.L. 1998. Species richness, spatial variation, and abundance of the soil seed bank of a secondary tropical rain forest. Biotropica 30:214-222. [ Links ]
Cavers P.B. and Benoit D.L. 1989. Seed banks in arable land. In: Leck M.A. and Parker V.T. Eds. Ecology of Soil Seed Banks, pp. 309-328, Academic Press, San Diego. [ Links ]
Cervantes-Gutiérrez V., Gama-Castro J., Hernández-Cárdenas G. and Meave del Castillo J.A. 2005. The land classification system of the San Nicolás Zoyatlán (S Mexico) Nahuatl indigenous community: a basis for a suitable parametric soil use proposal. Human Ecology Review 12:44-59. [ Links ]
Challenger A. 1998. Utilización y Conservación de los Ecosistemas Terrestres de México: Pasado, Presente y Futuro. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Universidad Nacional Autónoma de México and Agrupación Sierra Madre, Mexico City. [ Links ]
Chapman C.A. and Chapman L.J. 1999. Forest restoration in abandoned agricultural land: a case study from East Africa. Conservation Biology 13:1301-1311. [ Links ]
Colosi J.C., Cavers P.B. and Bough M.A. 1988. Dormancy and survival in buried seeds of proso millet (Panicum miliaceum). Canadian Journal of Botany 66:161-168. [ Links ]
Cubiña A. and Aide T.M. 2001. The effect of distance from forest edge on seed rain and soil seed bank in a tropical pasture. Biotropica 33:260-267. [ Links ]
Dalling J.W. and Hubbell S.P. 2002. Seed size, growth rate and gap microsite conditions as determinants of recruitment success for pioneer species. Journal of Ecology 90:557-568. [ Links ]
Dalling J.W., Muller-Landau H.C., Wright S.J. and Hubbell S.P. 2002. Role of dispersal in the recruitment limitation of neotropical pioneer species. Journal of Ecology 90:714-727. [ Links ]
Dalling J.W., Swaine M.D. and Garwood N.C. 1997. Soil seed bank community dynamics in seasonal moist lowland tropical forest, Panamá. Journal of Tropical Ecology 13:659-680. [ Links ]
Dalling J.W., Swaine M.D. and Garwood N.C. 1998. Dispersal patterns and seed bank dynamics of pioneer trees in moist tropical forest. Ecology 79:564-578. [ Links ]
Ekeleme F., Akobundu I.O., Isichei A.O. and Chikoye D. 2000. Influence of fallow type and land-use intensity on weed seed rain in a forest/savanna transition zone. Weed Science 48:604-612. [ Links ]
Ewel J., Berish C., Brown B., Price N. and Raich J. 1981. Slash and burn impacts on a Costa Rican wet forest site. Ecology 62:816-829. [ Links ]
Fajardo L., González V., Nassar J.M., Lacabana P., Portillo-Q. C.A., Carrasquel F. and Rodríguez J.P. 2005. Tropical dry forests of Venezuela: characterization and current conservation status. Biotropica 37:531-546. [ Links ]
Gallardo-Cruz J.A., Meave J.A., Pérez-García E.A. and Hernández-Stefanoni J.L. 2010. Spatial structure of plant communities in a complex tropical landscape: implications for β-diversity. Community Ecology 11:202-210. [ Links ]
Garwood N.C. 1983. Seed germination in a seasonal tropical forest in Panama: a community study. Ecological Monographs 53:159-181. [ Links ]
Garwood N.C. 1989. Tropical soil seed banks: a review. In: Leck M.A., Parker V.T. and Simpson R.L. Eds. Ecology of Soil Seed Banks, pp. 149-209, Academic Press, San Diego. [ Links ]
Guevara S., Moreno-Casasola P. and Sánchez-Ríos G. 2005. Soil seed banks in the tropical agricultural fields of Los Tuxtlas, Mexico. Tropical Ecology 46:219-227. [ Links ]
Grime J.P. 1979. Plant Strategies and Vegetation Processes. John Wiley & Sons, Chichester. [ Links ]
Grombone-Guaratini M.T. and Rodrigues R.R. 2002. Seed bank and seed rain in a seasonal semi-deciduous forest in south-eastern Brazil. Journal of Tropical Ecology 18:759-774. [ Links ]
Gross K.L. 1990. A comparison of methods for estimating seed numbers in the soil. Journal of Ecology 78:1079-1093. [ Links ]
Holl K.D. 1999. Factors limiting tropical rain forest regeneration in abandoned pasture: seed rain, seed germination, microclimate, and soil. Biotropica 31:229-242. [ Links ]
Hyatt L.A. and Casper B.B. 2000. Seed bank formation during early secondary succession in a temperate deciduous forest. Journal of Ecology 88:516-527. [ Links ]
Janzen D.H. 1988. Tropical dry forests: the most endangered major tropical ecosystem. In: Wilson E.O. Ed. Biodiversity, pp. 130-137, National Academy Press, Washington, D.C. [ Links ]
Jaramillo V.J., García-Oliva F. and Martínez-Yrízar A. 2010. La selva seca y las perturbaciones antrópicas en un contexto funcional. In: Ceballos G., Martínez L., García A., Espinoza E., Bezaury-Creel J. and Dirzo R. Eds. Diversidad, Amenazas y Áreas Prioritarias para la Conservación de las Selvas Secas del Pacífico de México, pp. 235-250, Fondo de Cultura Económica/Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Mexico City. [ Links ]
Kellman M.C. 1974. The viable weed seed content of some tropical agricultural soils. Journal of Applied Ecology 11:669-677. [ Links ]
Kennard D.K., Gould K., Putz F.E., Fredericksen T.S. and Morales F. 2002. Effect of disturbance intensity on regeneration mechanisms in a tropical dry forest. Forest Ecology and Management 162:197-208. [ Links ]
Kent M. and Coker P. 1994. Vegetation Description and Analysis: a Practical Approach. John Wiley and Sons, Chichester. [ Links ]
Khurana E. and Singh J.S. 2001. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: a review. Environmental Conservation 28:39-52. [ Links ]
Lebrija-Trejos E., Bongers F., Pérez-García E.A. and Meave J.A. 2008. Succesional change and resilience of a very dry tropical deciduous forest following shifting agriculture. Biotropica 40:422-431. [ Links ]
Lebrija-Trejos E., Pérez-García E.A., Meave J.A., Bongers F. and Poorter L. 2010. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 91:386-398. [ Links ]
Lebrija-Trejos E., Pérez-García E.A., Meave J.A., Poorter L. and Bongers F. 2011. Environmental changes during secondary succession in a tropical dry forest in Mexico. Journal of Tropical Ecology 27:477-489. [ Links ]
Lemenih M. and Teketay D. 2006. Changes in soil seed bank composition and density following deforestation and subsequent cultivation of a tropical dry Afromontane forest in Ethiopia. Tropical Ecology 47:1-12. [ Links ]
Lerdau M., Whitbeck J. and Holbrook N.M. 1991. Tropical deciduous forest: death of a biome. Trends in Ecology and Evolution 6:201-202. [ Links ]
Luzuriaga A.L., Escudero A., Olano J.M. and Loidi J. 2005. Regenerative role of seed banks following an intense soil disturbance. Acta Oecologica 27:57-66. [ Links ]
Maass J.M., Jordan C.F. and Sarukhán J. 1988. Soil erosion and nutrient losses in seasonal tropical agroecosystems under various management techniques. Journal of Applied Ecology 25:595-607. [ Links ]
Maass M., Búrquez A. Trejo I., Valenzuela D., González M.A., Rodríguez M. and Arias H. 2010. Amenazas. In: Ceballos G., Martínez L., García A., Espinoza E., Bezaury-Creel J. and Dirzo R. Eds. Diversidad, Amenazas y Áreas Prioritarias para la Conservación de las Selvas Secas del Pacífico de México, pp. 321-346, Fondo de Cultura Económica/Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Mexico City. [ Links ]
Marod D., Kutintara U., Tanaka H. and Nakashizuka T. 2002. The effects of drought and fire on seed and seedling dynamics in a tropical seasonal forest in Thailand. Plant Ecology 161:41-57. [ Links ]
Martins A.M. and Engel L.V. 2007. Soil seed banks in tropical forest fragments with different disturbance histories in southeastern Brazil. Ecological Engineering 31:165-174. [ Links ]
Maza-Villalobos S., Lemus-Herrera C. and Martínez-Ramos M. 2011. Successional trends in soil seed banks of abandoned pastures of a Neotropical dry region. Journal of Tropical Ecology 27:35-49. [ Links ]
McLaren K.P. and McDonald M.A. 2005. Seasonal patterns of flowering and fruiting in a dry tropical forest in Jamaica. Biotropica 37:584-590. [ Links ]
Meli P. 2003. Restauración ecológica en bosques tropicales. Veinte años de investigación académica. Interciencia 28:581-589. [ Links ]
Mena-Gallardo A. 2009. Variación del banco de semillas a lo largo de la sucesión secundaria en un bosque tropical caducifolio del sur de México. B.Sc Thesis, Universidad Nacional Autónoma de México, Mexico City, 55 p. [ Links ]
Miller P.M. 1999. Effects of deforestation on seed banks in a tropical deciduous forest of eastern Mexico. Journal of Tropical Ecology 15:179-188. [ Links ]
Miller P.M. and Kauffman J.B. 1998. Seedling and sprout response to slash-and-burn agriculture in a tropical deciduous forest. Biotropica 30:538-546. [ Links ]
Murphy P.G. and Lugo A.E. 1986. Ecology of tropical dry forest. Annual Review of Ecology and Systematics 17:67-88. [ Links ]
Oliver C.D. 1980. Forest development in North America following major disturbances. Forest Ecology and Management 3:153-168. [ Links ]
Paatela J. and Erviö L.-R. 1971. Weed seeds in cultivated soils in Finland. Annales Agriculturae Fenniae 10:144-152. [ Links ]
Pareja M.R. and Staniforth D.W. 1985. Seed-soil microsite characteristics in relation to weed seed germination. Weed Science 33:190-195. [ Links ]
Pérez E.M. and Santiago E.T. 2001. Dinámica estacional del banco de semillas en una sabana en los Llanos Centro-Orientales de Venezuela. Biotropica 33:435-446. [ Links ]
Pérez-García E.A., Meave J. and Gallardo C. 2001. Vegetación y flora de la región de Nizanda, Istmo de Tehuantepec, Oaxaca, México. Acta Botanica Mexicana 56:19-88. [ Links ]
Pérez-García E.A., Meave J.A., Villaseñor J.L., Gallardo-Cruz J.A. and Lebrija-Trejos E.E. 2010. Vegetation heterogeneity and life-strategy diversity in the flora of the heterogeneous landscape of Nizanda, Oaxaca, Mexico. Folia Geobotanica 45:143-161. [ Links ]
Pérez-Gutiérrez R., Solari L.A., Gómez-Tuena A. and Valencia V.A. 2009. El terreno Cuicateco: ¿cuenca oceánica con influencia de subducción del Cretácico Superior en el sur de México? Nuevos datos estructurales, geoquímicos y geocronológicos. Revista Mexicana de Ciencias Geológicas 26:222-242. [ Links ]
Pickett S.T.A. and McDonnell M.J. 1989. Seed bank dynamics in temperate deciduous forest. In: Leck M.A., Parker V.T. and Simpson R.L. Eds. Ecology of Soil Seed Banks, pp. 123-147, Academic Press, San Diego. [ Links ]
Poiani K.A. and Carter-Johnson W. 1988. Evaluation of the emergence method in estimating seed bank composition of prairie wetlands. Aquatic Botany 32:91-97. [ Links ]
Quintana-Ascencio P.F., González-Espinosa M., Ramírez-Marcial N., Domínguez-Vázquez G. and Martínez-Icó M. 1996. Soil seed banks and regeneration of tropical rain forest from milpa fields at the Selva Lacandona, Chiapas, Mexico. Biotropica 28:192-209. [ Links ]
Ramírez-Marcial N., González-Espinosa M. and Quintana-Ascencio P.F. 1992. Banco y lluvia de semillas en comunidades sucesionales de bosques de pino-encino de Los Altos de Chiapas, México. Acta Botanica Mexicana 20:59-75. [ Links ]
Rico-Gray V. and García-Franco J.G. 1992. Vegetation and soil seed bank of successional stages in tropical lowland deciduous forest. Journal of Vegetation Science 3:617-624. [ Links ]
Romero-Duque L.P., Jaramillo V.J. and Pérez-Jiménez A. 2007. Structure and diversity of secondary tropical dry forests in Mexico, differing in their prior land-use history. Forest Ecology and Management 253:38-47. [ Links ]
Russell-Smith J. and Lucas D.E.1994. Regeneration of monsoon rain forest in northern Australia: the dormant seed bank. Journal of Vegetation Science 5:161-168. [ Links ]
Sánchez-Azofeifa G.A., Quesada M., Rodríguez J.P., Nassar J.M., Stoner K.E., Castillo A., Garvin T., Zent E.L., Calvo-Alvarado J.C., Kalacska M.E.R., Fajardo L., Gamon J.A. and Cuevas-Reyes P. 2005. Research priorities for Neotropical dry forests. Biotropica 37:477-485. [ Links ]
Siebe C., Jahn R. and Stahr K. 1996. Manual para la Descripción y Evaluación Ecológica de Suelos en el Campo. Publicación especial 4. Sociedad Mexicana de la Ciencia de Suelo A.C., Chapingo. [ Links ]
Skoglund J. 1992. The role of seed banks in vegetation dynamics and restoration of dry tropical ecosystems. Journal of Vegetation Science 3:357-360. [ Links ]
StatSoft Inc. 2007. Statistica (Data Analysis Software System), version 8.0. <http://www.statsoft.com> [ Links ]
Trejo I. and Dirzo R. 2000. Deforestation of seasonally dry tropical forest: a national and local analysis in Mexico. Biological Conservation 94:133-142. [ Links ]
Uhl C., Buschbacher R. and Serrão E.A.S. 1988. Abandoned pastures in eastern Amazonia. I. Patterns of plant succession. Journal of Ecology 76:663-681. [ Links ]
Uhl C. and Clark K. 1983. Seed ecology of selected Amazon Basin successional species. Botanical Gazette 144:419-425. [ Links ]
Uhl C., Clark H., Clark K. and Maquirino P. 1982. Successional patterns associated with slash-and-burn agriculture in the Upper Río Negro region of the Amazon Basin. Biotropica 4:249-254. [ Links ]
Uhl C., Clark K., Clark H. and Murphy P. 1981. Early plant succession after forest cutting and burning in the Upper Rio Negro region of the Amazon Basin. Journal of Ecology 69:631-649. [ Links ]
Vieira D.L.M. and Scariot A. 2006. Principles of natural regeneration of tropical dry forests for restoration. Restoration Ecology 14:11-20. [ Links ]
Villaseñor J.L. and Espinosa-García F.J. 2004. The alien flowering plants of Mexico. Diversity and Distributions 10:113-123. [ Links ]
Vincent E.M. and Cavers P.B. 1978. The effects of wetting and drying on the subsequent germination of Rumex crispus. Canadian Journal of Botany 56:2207-2217. [ Links ]
Wijdeven S.M.J. and Kuzee M.E. 2000. Seed availability as a limiting factor in forest recovery processes in Costa Rica. Restoration Ecology 8:414-424. [ Links ]
Wild A. 1993. Soils and the Environment: An Introduction. Cambridge University Press, Cambridge. [ Links ]
Zar J.H. 1999. Biostatistical Analysis. Prentice-Hall, Upper Saddle River. [ Links ]
Zimdahl R.L. 1999. Fundamentals of Weed Science, Academic Press, San Diego. [ Links ]














