Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Botanical Sciences
versión On-line ISSN 2007-4476versión impresa ISSN 2007-4298
Bot. sci vol.90 no.3 México sep. 2012
Ecophysiology
Physiological responses of the Green manure, Vicia sativa, to drought
Respuestas fisiológicas del abono verde, Vicia sativa, a la sequía
Juan Tenopala1,2, Francisco Javier González3 and Erick de la Barrera1,4
1Centro de Investigaciones en Ecosistemas, Universidad Nacional Autónoma de México, Campus Morelia, Michoacán, México.
2Licenciatura en Ciencias Ambientales, Universidad Nacional Autónoma de México, Campus Morelia, Michoacán, México.
3Coordinación para la Innovación y Aplicación de la Ciencia y la Tecnología, Universidad Autónoma de San Luis Potosí, San Luis Potosí, México.
4Autor para la correspondencia: erick@cieco.unam.mx
Received: October 5, 2011
Accepted: February 27, 2012
Abstract
Cover crops and green manures have been adopted in rainfed agriculture for providing soil protection between cultivation seasons and for increasing soil nutrient and organic matter content. A greenhouse experiment was conducted to evaluate physiological responses of the green manure Vicia sativa to drought. The gravimetric leaf water of content of 80% did not decrease during 24 days of water withholding. Gas exchange was very sensitive to drought. For instance, net CO2 uptake of 7.8 ± 0.17 µmol m-2 s-1 started decreasing at eight days of water withholding but recovered when the plants were re-watered. While total chlorophyll was not significantly degraded for the droughted plants, these individuals accumulated 1,000-fold the proline of the control, which amounted to 0.41 ± 0.09 µmol g-1. Raman spectroscopy revealed differences in the accumulation of metabolites at 24 days of water withholding between well-watered and droughted plants, including a higher content of abscisic acid, gibberellic acid, indoleacetic acid, and zeatin. Vicia sativa can tolerate month-long droughts and can be considered as a useful alternative for increasingly arid locations.
Key words: agroecology, isohydric species, proline, Raman spectroscopy, vetch.
Resumen
Los cultivos de cobertura y abonos verdes se utilizan en agricultura de temporal para proporcionar protección contra la erosión de los suelos entre temporadas de cultivo y para aumentar el contenido de nutrientes y materia orgánica. Se realizó un experimento en el invernadero para evaluar algunas respuestas fisiológicas del abono verde Vicia sativa a la sequía. El contenido gravimétrico de agua en las hojas, que fue de 80%, no disminuyó durante 24 días de suspensión del riego. Por su parte, el intercambio de gases fue muy sensible a la sequía. Por ejemplo, la asimilación neta de CO2 de 7.8 ± 0.17 µmol m-2 s-1 comenzó a disminuir a los ocho días de suspender el riego, pero se recuperó en cuanto las plantas fueron vueltas a regar. Mientras que la clorofila total no se degradó en las plantas sin riego, estos individuos acumularon 1,000 veces más prolina que el control, cuya concentración del aminoácido fue de 0.41 ± 0.09 µmol g-1. Mediciones de espectroscopía por esparcimiento Raman revelaron diferencias en la acumulación de metabolitos entre el control y las plantas sin riego a los 24 días de suspensión del riego, incluyendo un aumento en el contenido de ácido abscísico, ácido giberélico, ácido indolacético y zeatina. Vicia sativa es capaz de tolerar sequías con duración de un mes y puede ser una alternativa útil para la agricultura en sitios cada vez más secos.
Palabras clave: agroecología, ebo o janamargo, especies isohídricas, espectroscopía Raman, prolina.
Cover crops provide plant cover in agricultural lands during intercrop periods, reducing soil erosion and by incorporating nutrients and organic matter to the soil (Brady and Weil, 1996). Various legume cover crops also act as green manures by increasing soil nitrogen content through biological fixation by the symbiotic bacteria Rhizobium. This nitrogen supplementation can contribute to reducing the consumption of synthetic fertilizers, which greatly increase the economic and energetic costs of food production and generate environmental pollution (Ellis and Pontius, 2010).
Vicia sativa L. is a nitrogen-fixing legume that is widely used as green manure and cover crop (Uzum et al., 2011). It is utilized in rain fed agriculture, where it is planted during the dry months utilizing residual soil humidity and late rains. Its use as a green manure reduces the consumption of chemical fertilizers without affecting crop yield and reduces contaminant leaching to water bodies (Salmerón et al., 2011). Another consequence of its use is the reduction of cultivation associated costs, leading to its adoption in small scale agricultural operations, which in Mexico are usually rainfed and for self-consumption (De la Tejera-Hernández and García-Barrios, 2008; Orozco-Martinez et al., 2012).
As drought progresses, the soil water potential decreases and the capacity of plants to take up water from the soil is reduced (Munns, 2002; Chaves et al., 2003; Hamdy et al., 2003; Flexas et al., 2006). An initial response of plants to drought is a reduction in net CO2 uptake and a relative increase in the rate of respiration (Flexas et al., 2005, 2006). Plants can also respond to an increasingly negative soil water potential by accumulating osmotically active solutes, such as proline, which is accumulated in larger amounts by drought-resistant cultivars of various species than in their less tolerant counterparts (Hanson, 1976; Xue et al., 2008; DaCosta and Huang, 2009). It has been well documented that a buildup of the phytohormone abscisic acid is a trigger for plant responses to drought, but the role and the behavior of other phytohormones are not well understood for plants growing in drying soil (Nobel and de la Barrera, 2002; Srivastava, 2002). However, the concentrations of gibberellic acid, indoleacetic acid, and zeatin have been found to decrease for droughted soybean (Zhang et al., 2011).
Some physiological responses of Vicia sativa to a drought were assessed in a greenhouse experiment in order to determine the potential suitability of this green manure as a cover crop in semi-arid lands or in regions with a decreasing water availability resulting from climate change or environmental degradation at the landscape level. In particular, water relations of gas exchange, chlorophyll integrity, and proline accumulation were considered. Also, the novel technique of Raman scattering spectroscopy was utilized to corroborate leaf content of proline and to determine phytohormone levels.
Materials and methods
Plant material and treatments. Seeds of Vicia sativa L. (Fabaceae) were obtained from a local vendor (Casa Treviño, Guadalajara, Jalisco). Seeds were sown on 10 January 2011 in nursery bags (volume of 3.8 l) containing a locally available growth medium composed of coconut fiber, tree bark, and agrolite (CreciRoot, Sistemas Agrotec, Uruapan, Michoacán, México; when watered to saturation this substrate drains within 10 min to a field capacity of 50% by volume), which were kept in a greenhouse at the Centro de Investigaciones en Ecosistemas, Universidad Nacional Autónoma de México, Morelia, Michoacán (19º 38' 55.9" N; 13º 13' 43.7" W; 1,967 m), fitted with a weather station. At this site the air temperature inside the greenhouse averaged 17 ºC during the experiment, ranging from 2 ºC to 36 ºC. In turn, the relative humidity averaged 59%, ranging from 5% to 97%, and the photosynthetic photon flux (wavelengths of 400 to 700 nm) reaching the plants amounted to 18.6 mol m-2 day-1.
Bags were watered every four days to saturation and allowed to drain to field capacity (50% by volume). After approximately two months of development, a drought experiment was established (plants had an average height of 60 ± 20 cm, reflecting the genetic variability of the landrace of Vicia sativa that was utilized) on 28 February 2011, when irrigation was suspended for a group of five randomly selected individuals and continued for a control group of the same size. Various physiological parameters were monitored periodically to determine the response of the experimental group. Finally, irrigation was resumed on 25 March 2011 to determine the kinetics of drought recovery.
Tissue water content. Plant water status during the drought and recovery experiment was determined as the volumetric water content of leaves. In particular, a randomly chosen leaflet from a fully developed leaf was weighed and dried in a gravity convection oven at 70 ºC until constant weight was obtained, before determining its dry weight. Water content was calculated as 100 × [(fresh mass - dry mass) / fresh mass].
Gas exchange. Gas exchange, i.e., stomatal conductance, transpiration, and net CO2 uptake, were measured for randomly chosen, fully developed leaves with a CI-340 Hand-Held photosynthesis system (CID Instruments, Camas, Washington, U.S.A.). Measurements, which were taken by triplicate for each one of five plants per treatment, were conducted at 16:00 h every four days. A transparent leaf cuvette was attached to an open setting of the photosynthesis measuring instrument to ensure that the microenvironmental conditions within the chamber were as close as possible to those prevailing in the greenhouse. The time of measurement was determined after a set of preliminary measurements conducted during the day for which the times for gas exchange maxima were at 10:00 h and 16:00 h.
Metabolite quantification. Changes in the tissular concentration of total chlorophyll during drought and recovery were measured according to Lichtenthaler (1987). Three randomly chosen leaflets from the same leaves utilized for gas exchange measurements were separately weighed and macerated with a cold (3 ºC) aqueous solution of acetone (80% v/v) and brought up to a final volume of 3.0 ml. Absorbance at 665 nm and 645 nm was measured with an EZ 301 Spectrometer (Perkin Elmer, Waltham, Massachusetts, U.S.A.). The chlorophyll concentrations of the three leaflets described above were averaged for each one of five individuals per treatment.
Proline accumulation in the leaf tissue of Vicia sativa was measured during drought and recovery by spectrophotometry (Bates, 1973) for three randomly chosen leaflets from each one of the leaves that were utilized for gas exchange measurements. In particular, 1 g of fresh leaf tissue was frozen with liquid nitrogen and homogenized in a porcelain mortar with 10 ml of sulfosalicilic acid (3% v/v) and filtered in a funnel with No. 1 filter paper. From the filtrate, 2 ml were taken and placed in a test tube with 2 ml of glaciar acetic acid and a reactive solution of ninhidrin. The reactive solution of ninhidrin was obtained by mixing 2.5 g of ninhidrin with 60 ml of glaciar acetic acid (99% v/v), after incubation at 100 ºC for 15 min, 40 ml of orthosphosphoric acid were added. This mixture was stirred in a vortex for 15 seconds, incubated for 1 h at 100 ºC, and quickly cooled in ice. Finally, 4 ml of toluene were added and stirred in a vortex for 30 seconds. Absorbance at 520 nm was measured for the resulting colored compound.
Raman scattering measurements were performed on one leaflet per plant that had been frozen in liquid nitrogen and stored in the dark at -80 ºC until utilized. All measurements were conducted at room temperature with a Raman Systems R3000 spectrometer (Ocean Optics, Dunedin, Florida, U.S.A.) fitted with a 785 nm laser diode, a spectral resolution of 8 cm-1, and a laser power of 90 mW. The measurements were made in the 200 to 1,800 cm-1 spectral range and the instrument was calibrated using a Teflon standard before each round of measurements. The measured spectra were pre-processed by subtracting a fifth grade polynomial to the raw spectra applying the Vancouver fluorescence removal algorithm (Zhao et al., 2007) in order to remove the background near infrared fluorescence and leave the pure Raman signal. A principal components analysis was performed on the clean spectra as an initial characterization of the metabolomic signatures of well irrigated and droughted plants (Jollifee, 2002; González et al., 2011a).
Finally, the relative tissue content of proline and the phytohormones abscisic acid, gibberellic acid, indoleacetic acid, and zeatin, were estimated with a vector correlation between previously determined reference spectra for each metabolite and the clean spectra obtained for the leaves. This procedure has been successfully used to determine the concentration of proteins in human skin (González et al., 2011b). The Vancouver algorithm, the principal components analysis, and the vector correlation were performed with Mathematica (Wolfram Research, Champaign, Il, USA).
Experimental design and statistical analyses. The present work was conducted under a completely randomized design. Data were usually analyzed with repeated measures ANOVAs (Sokal and Rohlf, 1995). Post-hoc pair-wise comparisons were conducted with Tukey tests, except for data from Raman spectroscopy, for which Student t-tests were performed at 24 days of water withholding. Statistical analyses were conducted with SigmaStat 3.5 (Systat Software, Point Richmond, California, U.S.A.). Data are shown as mean ± 1 s.e. (n = sample size), unless indicated otherwise.
Results
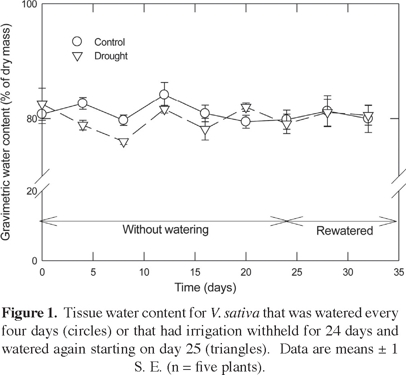
Tissue water content for both treatments remained constant over the course of the experiment, averaging 80% by mass for both the well watered control and the droughted individuals (P = 0.256 from a Repeated Measures ANOVA; Figure 1).
Stomatal conductance averaged 80.33 ± 7.08 mmol m-2 s-1 over the course of the experiment for plants of Vicia sativa that were well watered (Figure 2A). For the case of the droughted plants, stomatal conductance was lower than for the control (P < 0.001), ranging from 41% lower at 12 days without irrigation to 55% lower at 24 days without irrigation. Stomatal conductance recovered to rates similar to the control at 8 days after resuming irrigation.
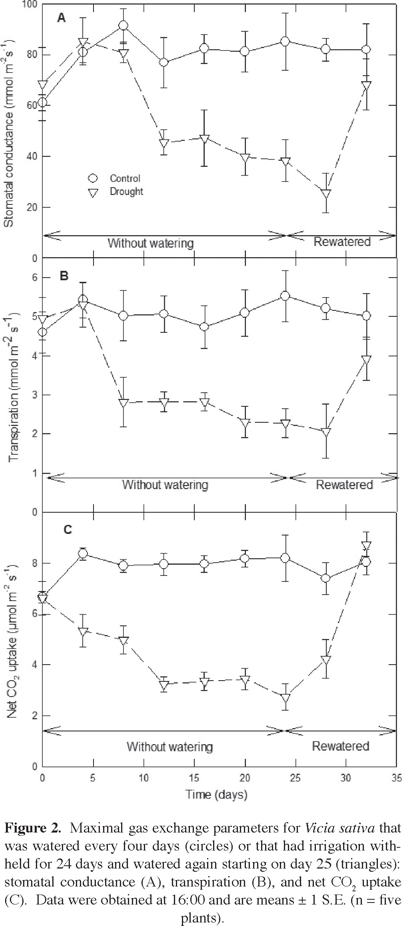
Transpiration averaged 5.07 ± 0.53 µmol m-2 s-1 over the course of the experiment for plants of Vicia sativa that were well watered (Figure 2B). For the case of the droughted plants, transpiration was lower than the control (P < 0.001), ranging from 44% lower at 8 days without irrigation to 59% lower at 24 days without irrigation. Transpiration recovered to rates similar to the control at 8 days after resuming irrigation.
Net CO2 uptake averaged 7.82 ± 0.44 µmol m-2 s-1 over the course of the experiment for plants of Vicia sativa that were well watered (Figure 2C). For the case of the droughted plants, it was lower than for the control (P < 0.001), ranging from 36% lower at 4 days without irrigation to 66% lower at 24 days without irrigation. At 4 days after resuming irrigation, the total CO2 uptake started to increase, returning to levels similar to those of the control by 8 days after resuming irrigation.
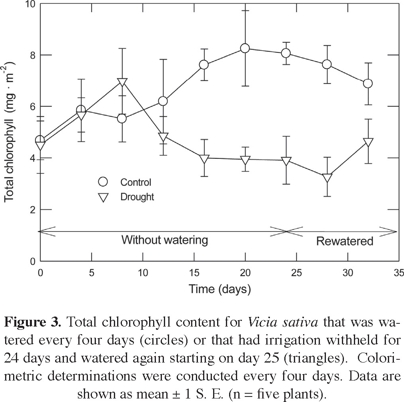
Total chlorophyll content averaged 6.74 ± 0.95 mg m-2 over the course of the experiment for Vicia sativa (Figure 3). While a trend can be observed of a lower chlorophyll content for droughted plants, the differences were not significant during the experiment (P = 0.472).
The proline levels averaged 0.64 ± 0.37 µmol g-1 (fresh weight basis) over the course of the experiment for plants of Vicia sativa that were well watered (Figure 4A). For the case of the droughted plants, prolina content became 1,000-fold higher at 12 days without irrigation and remained high while water was withheld (P < 0.001). At 4 days after resuming irrigation, the tissue concentration of proline started to decrease, returning to levels similar to those of the control by 8 days after resuming irrigation.
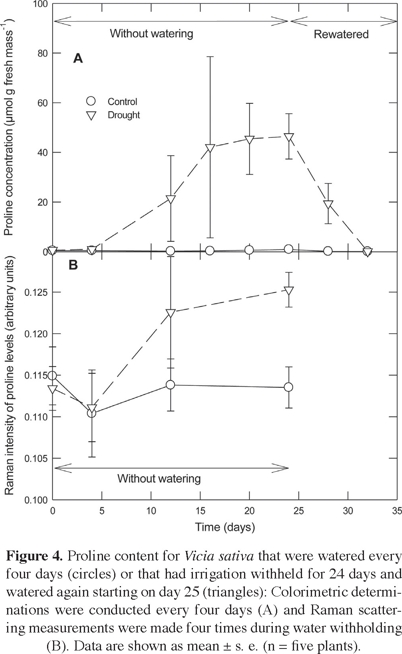
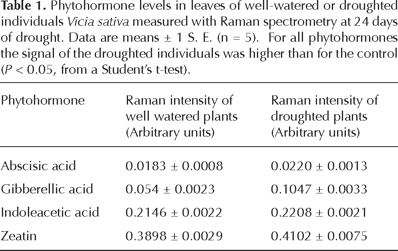
A principal components analysis of Raman spectra for Vicia sativa revealed that the first component explained 99.56% of the variance. A more detailed analysis of this first principal component indicated that its magnitude for droughted plants was similar to the control over most of the experiment (Figure 5). However, a significant difference was detected at 24 d of water withholding (P < 0.05). Moreover, a higher accumulation of proline was confirmed for droughted individuals from a vector correlation of leaf vs. reference Raman spectra (Figure 4B). Similarly, the tissue levels of abscisic acid (20% higher; P < 0.05 from a Student t-test), gibberellic acid (93%), indoleacetic acid (3%), and zeatin (5%), were higher at 24 days of water withholding for droughted individuals than for the well watered control (Table 1).
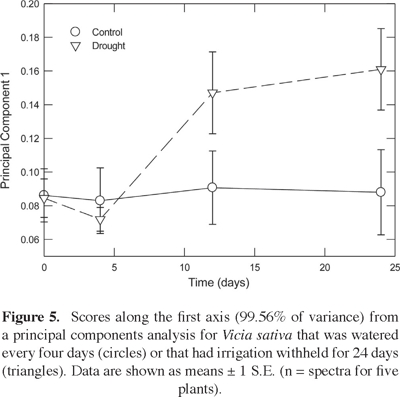
Discussion
Drought led to a reduction in gas exchange and to an accumulation of the osmotically active amino acid, proline, but not to chlorophyll degradation for Vicia sativa, a cover crop that is also utilized as green manure, which relies on residual soil humidity. However, over the course of the 24 days spanning water withholding, tissue water content did not decrease. This is consistent with the behavior of an "isohydric" species, for which the leaf water status does not correlate with the soil water status (Lambers et al., 1998). For such species, the roots respond to a decreasing water potential by producing abscisic acid, which in turn closes stomata before a substantial water loss occurs, helping the plant resist drought (DaCosta and Huang, 2009). Such is the case for Hylocereus undatus, Vigna sinensis, and Zea mays, among other species, for which an interruption of irrigation does not affect leaf water potential because the stomatal conductance declines before any adverse effects on water shortage reaches the leaves (Lambers et al., 1998; Nobel and de la Barrera, 2002).
Also consistent with isohydric behavior, gas exchange for Vicia sativa rapidly decreased after water withholding. In particular, transpiration halved at 8 days of drought, while stomatal conductance decreased by 1/3 at 12 days of drought. This is consistent with a response to drying soil. For example, for a silica sand/vermiculite mixture of a similar water-holding properties as the substrate utilized here, the water potential of a 10 cm-deep column reaches the permanent wilting point of -1.5 MPa at ca. 5 days after water withholding, leading to a decrease of 1/3 in the stomatal conductance of a succulent crop, while the water status of the plants does not change (Lambers et al., 1998; Nobel and de la Barrera, 2002).
The first effect of drought on plant productivity is a reduction of the net CO2 uptake rate (Taiz and Zeiger, 2002). Indeed, such assimilation was the most sensitive parameter of gas exchange to drought for Vicia sativa. The fact that a significant reduction of carbon assimilation occurred four days earlier than a reduction of transpiration could be reflecting the physiological water requirements of photosynthesis, i.e., water is the source of electrons for photosynthesis (Taiz and Zeiger, 2002). A faster reduction of photosynthesis than of transpiration also occurs for Dorycnium hirsutum, Gossypium hirsutum, and Phaseolus vulgaris (Samarakoon and Gifford, 1996; Mayashita et al., 2004; Moreno et al., 2008). While the assimilation rates of droughted plants were less than half of those of the control individuals, no permanent damage to the photosynthetic apparatus of V. sativa appeared to occur over the duration of the experiment, as suggested by the recovery of the carbon assimilation rate within one week of resuming irrigation and the lack of significant changes in chlorophyll content. It is likely that a longer drought would have led to permanent photosynthetic damage, as it occurs for Poa pratensis (14 days of drought until permanent damage; Jiang and Huang, 2001) or Festuca arundinacea (24 days of drought until death; Wang and Huang, 2004). In any case, for V. sativa, whose lifecycle spans a mere 120 days, the lethal effects of a drought of about a month in duration may be overpowered by the onset of senescence. In this case, the crop can be utilized in increasingly arid locations as long as an initial water pulse of sufficient magnitude is available, whose magnitude requires further investigation.
While transpiration was reduced substantially following water withholding, it did not cease. Water from the substrate might have been extracted by the plant by further reducing its osmotic potential, e.g., via the accumulation of proline and other osmolites, in order to sustain the constant gravimetric tissue water content that was observed. Water deficit may have induced a dramatic increase of proline concentration in response osmotic stress because the accumulation of this amino acid in the vacuoles mediates osmotic adjustments (Taiz and Zeiger, 1998). In addition, proline reduces stress-induced cellular acidification and provides energy for recovery from drought via prime oxidative respiration (Hare and Cress, 1997). Here, an increase in proline levels was detected the same day that stomatal conductance started to decrease, confirming for V. sativa, as it occurs for other plant species, that the proline concentration can be utilized as an indicator of plant responses to water stress.
The substantial increase on phytohormones observed at 24 days of suspending irrigation seemed to reflect plant responses to water withholding, although the specific drought relations of each phytohormone are not well understood (Taiz and Zeiger, 1998; Srivastava, 2002). An exception is abscisic acid, which is a known trigger of physiological responses to drought, such as the ones reported here (Taiz and Zeiger, 1998; Nobel and de la Barrera, 2002). However, our results are opposite to a decrease of the content of gibberellins, indoleacetic acid, and zeatin, reported for water-stressed soybean (Zhang et al., 2011).
Vicia sativa was able to withstand a month-long drought by reducing its transpirational water loss and accumulating osmotically active solutes, which enabled this green manure, cover crop to quickly recover its physiological processes once irrigation was resumed. The extent and velocity of physiological recovery from drought depends on the degree of recovery of photosynthesis (Flexas et al., 2006). Here, the fact that photosynthetic pigments did not decrease significantly, probably allowed the plants to have a rapid recovery of physiological processes. This suggests that V. sativa can be planted in increasingly arid locations, especially when its short lifespan is considered. Future research should validate these findings in the field and explore such attributes as the resistance to drought at different developmental stages, the maximum duration of drought that still enables the development of the crop, and the minimum initial amount of soil humidity required. While the plant responses measured with Raman spectroscopy were not detected as early as with conventional spectroscopic measurements, this methodology could be utilized for real-time in-vivo physiological studies and can be expected to advance our understanding of plant metabolomics in the near future.
Acknowledments
This project was funded by the Dirección General del Personal Académico, Universidad Nacional Autónoma de México (UNAM; PAPIIT IN224910), and institutional funds of the Centro de Investigaciones en Ecosistemas, UNAM. We thank Mauricio Quesada, Jorge E. Schondube, and Ken Oyama for access to greenhouse and laboratory facilities, Manuel Gutiérrez Hernández who conducted Raman spectroscopy measurements, Víctor Rocha and Gumercindo Sánchez for logistical support, and useful discussions with Zue Guerrero, Edison Díaz and Rodrigo Orozco. A previous version of this work was evaluated by Horacio Cano, Mayra Gavito, Roberto Lindig, and Ana Noguez, as part of the requirements to obtain the degree of Licenciado en Ciencias Ambientales at UNAM.
Literature cited
Bates L.S., Waldern R.P. and Teare I.D. 1973. Rapid determination of free proline for water-stress studies. Plant and Soil 39:205-207. [ Links ]
Brady N.C. and Weil R.R. 1996. The Nature and Properties of Soils. Prentice Hall. Upper Saddle River. [ Links ]
Chaves M.M., Maroco J.P. and Pereira J.S. 2003. Understanding plant responses to drought -from genes to the whole plant. Functional Plant Biology 30:239-264. [ Links ]
DaCosta M. and Huang B. 2009. Physiological adaptations of perennial grasses to drought stress In: de la Barrera E. and Smith W.K. Eds. Perspectives in Biophysical Plant Ecophysiology: A Tribute to Park S. Nobel, pp. 169-190, Universidad Nacional Autónoma de México, San Antonio. [ Links ]
De la Tejera-Hernández B. and García-Barrios R. 2008. Agricultura y estrategias de formación de ingreso campesinas en comunidades indígenas forestales oaxaqueñas. In: García-Barrios R., De la Tejera-Hernández B. and Appendini K. Eds. Instituciones y Desarrollo: Ensayos sobre la Complejidad del Campo Mexicano, pp. 65-103, Universidad Nacional Autónoma de México, México, D.F. [ Links ]
Ellis E. 2010. Land-use and land-cover change. In: Cleveland C.J. and Pontius R. Eds. Encyclopedia of Earth. Washington, D.C.: Environmental Information Coalition, National Council for Science and the Environment. <http://www.eoearth.org/article/Land-use_and_land-cover_change> (accessed October 1, 2011) [ Links ]
Flexas J., Bota J., Galmés J., Medrano H. and Ribas-Carbó M. 2006. Keeping a positive carbon balace under adverse conditions: responses of photosyntesis and respiration to water stress. Physiologia Plantarum 127:343-352. [ Links ]
Flexas J., Galmés J., Ribas-Carbó M. and Medrano H. 2005. The effects of drought in plant respiration. In: Lambers H. and Ribas-Carbo M. Eds. Plant Respiration: from Cell to Ecosystem, pp. 85-94, Kluwer Academic Publishers, Dordrecht. [ Links ]
González F.J., Alda J., Moreno-Cruz B., Martínez-Escanamé M., Ramírez-Elías M.G., Torres-Álvarez B. and Moncada B. 2011a. Use of Raman spectroscopy in the early detection of fillaggrin-related atopic dermatitis. Skin Research and Technology 17:45-50. [ Links ]
González F.J., Valdés-Rodríguez R., Ramírez-Elías M.G., Castillo-Martínez C., Saavedra-Alanís V.M. and Moncada B. 2011b. Noninvasive detection of filaggrin gene mutations using Raman spectroscopy. Biomedical Optics Express 2:3363-3366. [ Links ]
Hamdy A., Ragab R. and Scarascia-Mugnozza E. 2003. Coping with water scarcity: water saving and increasing water productivity. Irrigation and Drainage 52:3-20. [ Links ]
Hanson A.D., Nelsen C.E. and Everson E.H. 1976. Evaluation of free proline accumulation as an index of drought resistance using two contrasting barley cultivars Crop Science 17:720-726. [ Links ]
Hare P.D. and Cress W.A. 1997. Metabolic implications of stress-induced proline accumulations in plants. Plant Growth Regulation 21:79-102. [ Links ]
Jiang Y. and Huang B. 2001. Drought and heat stress injury of two cool-seasons turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Science 41:436-442. [ Links ]
Jolliffee I.T. 2002. Principal Component Analysis. Springer-Verlag, New York. [ Links ]
Lambers H., Chapin F.S. and Pons T.L. 1998. Plant Physiological Ecology. Springer, New York. [ Links ]
Lichtenthaler H.K. 1987. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In: Packer L. and Douce R. Eds. Methods in Enzymology Vol. 148: Plan Cell Membranes, pp. 350-382, Academic Press, San Diego. [ Links ]
Mayashita K., Tanakamaru S., Maitani T. and Kimura K. 2004. Recovery response of photosynthesis, transpiration and conductance in kidney bean following drought stress. Environmental and Experimental Botany 53:205-214. [ Links ]
Moreno M., Gulias J., Lazaridou M., Medrano H. and Cifre J. 2008. Ecophysiological strategies to overcome water deficit in herbaceous species under Mediterranean conditions. Options Méditerranéennes Series A 79:247-257. [ Links ]
Munns R. 2002. Comparative physiology of salt and waterstress. Plant Cell and Environment 25:239-250. [ Links ]
Nobel P.S. and de la Barrera E. 2002. Stem water relations and net CO2 uptake for a hemiepiphytic cactus during short-term drought. Environmental and Experimental Botany 48:129-137. [ Links ]
Orozco-Martínez R., del-Val E., Lindig-Cisneros R., Paz H., Quesada M. and de la Barrera E. 2012. Evaluation of three organic fertilizers for growing the widely cultivated crop Cucurbita pepo L. African Journal of Agricultural Research 7:1087-1097. [ Links ]
Sokal R.R. and Rohl F.J. 1995. Biometry: the Principles and Practice of Statistics in Biological Research. W.H. Freeman, New York. [ Links ]
Salmerón M., Isla R. and Cavero J. 2011. Effect of winter cover crop species and planting methods on maize yield and N availability under irrigated mediterranean conditions. Field Crops Research 123:89-99. [ Links ]
Samarakoon A.B. and Gifford R.M. 1996. Water use and growth of cotton in response to elevated CO2 in wet drying soil. Australian Journal of Plant Physiology 23:63-74. [ Links ]
Srivastava L.M. 2002. Plant Growth and Development: Hormones and Environment. Academic Press, San Diego. [ Links ]
Taiz L. and Zeiger E. 1998. Plant Physiology. Sinauer, Sunderland. [ Links ]
Uzum A, Gücer S. and Acikgoz E. 2011. Common vetch (Vicia sativa L.) germplasm: Correlations of crude protein and mineral content to seed traits. Plant Foods and Human Nutrition 66:254-260. [ Links ]
Wang Z. and Huang B. 2004. Physiological recovery of Kentucky bluegrass from simultaneous drought and heat stress. Crop Science 44:1729-1736. [ Links ]
Xue X., Liu A. and Hua X. 2008. Proline accumulation and transcriptional regulation of proline biosynthesis and degradation in Brassica napus. BMB Reports 42:28-34. [ Links ]
Zhang J., Smith D.L., Liu W., Chen X. and Yang W. 2011. Effects of shade and drought stress on soybean hormones and yield of main-stem and branch. African Journal of Biotechnology 10:14392-14398. [ Links ]
Zhao J., Lui H., McLean D.L. and Zeng H. 2007. Automated autofluorescence background subtraction algorithm for biomedical Raman spectroscopy. Applied Spectroscopy 61:1225-1232. [ Links ]














