Abbreviations
TURB: Transurethral resection of bladder
NMIBT: Non-muscle invasive bladder tumor (NMIBT)
Re-TURB: re-Transurethral resection of bladder / second TURB
BC: Bladder Cancer
MMC: mitomicyn-C
AUC: area under curve
Introduction
Bladder cancer (BC) is the 10th most commonly diagnosed cancer worldwide, and the 7th if only male population is considered; with an incidence rate in Europe of 20 per 100,000 person/years for men and 2.6 for women.1
Approximately 80% of BC presents as non-muscle-invasive bladder tumors (NMIBT), tumors confined to the mucosa (Ta o CiS) or submucosa (T1). Transurethral resection of bladder (TURB) is considered the gold standard treatment of NMIBT, which can be followed by adjuvant intravesical treatment depending on the risk stratification.1
According to the literature, residual tumor is found in almost 60% of Ta and T1 stage, so the re-TURB is nowadays a fundamental step to achieve a complete resection and appropriately stratify the tumor.2,3 Up to 2016, the European Association of Urology (EAU) Guidelines recommended to carry out a second TURB in patients with: first incomplete TURB, absence of muscular layer in the specimen after initial resection, and in cases of high grade or T1 NMIBT. However, since 2017, high grade indication has been removed from the EAU Guidelines.1 The American Urological Association guidelines, on the other hand, do recommend considering performing a second resection in high-grade Ta tumors.4
The re-TURB implies and additional expense for the health system, and as an invasive procedure it is not free of complications: from pain, retention or hematuria to bladder perforation or urinary sepsis; and other long-term complications, such as urethral strictures.5
The objective of this study is to design a predictive model of residual tumor in re-TURB, to optimize patient selection and avoid unnecessary surgeries.
Material and methods
We performed a retrospective analysis of 413 patients with NMIBT, diagnosed in our center between January 2010 and December 2021, and treated with a macroscopically complete initial TURB and identification of muscular layer in the pathological specimen. Treatment was completed by a second TURB performed at 2 to 6 weeks, according to current criteria of the EAU Guidelines. All surgeries were performed using a bipolar resector.
The pathological analysis was carried out by two reference pathologists from our center. Tumor staging was carried out following the 7th edition of the TNM classification, and histological grade was classified according to the 2004/2016 WHO classification system.1,6
A single intravesical instillation of mitomycin-C (MMC) was administered in the first 24 hours after the first TURB in some patients following the EAU and EORCT recommendations: in primary tumors or intermediate-risk recurrent tumors with a prior recurrence rate of less than or equal to one recurrence per year and those with a 2006 EORTC recurrence score <5.1,7
Early complications (in the first 30 days) were classified according to the Clavien-Dindo classification.5
We have identified predictive variables of residual tumor by means of univariant and multivariant analysis using logistic regression. Statistical significance has been considered with p value <0.05. The evaluation of the exactitude of the predictive model has been made using AUC (area under curve). All statistical analysis have been made using statistical program IBM® SPSS® statistics v-25.
Results
The baseline characteristics of the patients at the initial and the second TURB are detailed in Table 1.
Table 1 Baseline characteristics of the patients
| Variable | n=413 |
|---|---|
| Gender | |
| Male | 358 (86.7%) |
| Female | 55 (13.3%) |
| Stage | |
| Ta | 64 (15.5%) |
| T1 | 349 (84.5%) |
| Histological grade | |
| Low grade | 72 (17.4%) |
| High grade | 341 (82.6%) |
| Recurrence | |
| Primary | 267 (64.6%) |
| Recurrent | 146 (35.3%) |
| Number of lesions | |
| Single | 187 (45.3%) |
| 2-7 | 189 (45.8%) |
| 8 or more | 37 (9%) |
| Size | |
| < 3cm | 302 (73%) |
| >/= 3cm | 111 (26.9%) |
| Early instillation MMC | |
| Yes | 161 (39%) |
| No | 252 (413%) |
| Complications | |
| Anemizing hematuria | 17 (4.1%) |
| ITU | 7 (1.7%) |
| Bladder perforation | 3 (0.7%) |
| Tumor detection rate after second TURB (initial Ta+T1) | |
| No residual tumor | 297 (71.9%) |
| Ta | 51 (12.3%) |
| T1 | 42 (10.2%) |
| CiS | 14 (4.3%) |
| T2 | 9 (2.2%) |
| Tumor detection rate after second TURB in inital Ta Stage | |
| No residual tumor | 48 (75%) |
| Ta | 9 (14.1%) |
| T1 | 2 (3.1%) |
| CiS | 5 (7.8%) |
| Tumor detection after second TURB in intial T1 stage | |
| No residual tumor | 249 (71.3%) |
| Ta | 42 (12%) |
| T1 | 40 (11.5%) |
| CiS | 9 (2.6%) |
| T2 | 9 (2.6%) |
| Histological grade after second TURB | |
| Low grade | 46 (39.7%) |
| High grade | 70 (60.3%) |
The median age in the series was 74 (38-91) years old, and 86.7% (n=358) patients were male.
Tumors that required second TURB were mostly primary (64.6%), stage T1 (84.5%), high grade (82.6%), multifocal (54.8%) and with a size less than 3 cm (73.1%).
37 patients (6.5%) had early complications: 17(4.1%) hematuria, 7(1.7%) urinary tract infections, and 3 (0.7%) bladder perforation. All complications were managed conservatively (Clavien-Dindo <IIIb).
After analyzing pathological results, residual tumor was found in 28.1% of second TURB (n=116) and T2 tumor was re-stratified in 2.2% (n=9) cases (none of them with initial pathological anatomy T1). Considering the initial T stage, residual tumor was found in 25% (n=16) of the Ta patients: 56.25% (n=9) residual Ta tumor, 31.25% (n=5) residual Cis and 12.5% (n=2) residual T1. On the other hand, residual tumor was found in 28.7% (n=100) of the initial T1 patients: 42% (n=42) residual Ta, 40% (n=40) residual T1, 9% (n=9) Cis and other 9% (n=9) T2.
After final multivariant analysis we identified independent predictive factors of residual tumors (Table 2): recurrence tumor (OR 1.87; IQ 1.14-3.06, p=0.01) and multifocality tumors (OR 2.11; IQ 1.31-3.4; p=0.002), thus doubling the risk of residual tumor in re-TURB. High-grade shows a trend to statistical signification as risk factor (p=0.07) and early administration of mitomycin-C behaved as an independent protector factor (OR 0.40; IQ 0.3-0.81; p=0.006).
Table 2 Multivariate risk factors for residual disease at the time of second TURB
| Variable | OR | IC95% | P |
| Primary tumor | 1.26 | 0.65-2.46 | 0.49 |
| Ta | |||
| T1 | |||
| Grade | 1.84 | 0.95-3.56 | 0.07 |
| Low | |||
| High | |||
| Recurrence | 1.87 | 1.14-3.06 | 0.01 |
| Primary | |||
| Recurrent | |||
| Multifocality | 2.11 | 1.31-3.40 | 0.02 |
| Size | 1.49 | 0.88-2.52 | 0.13 |
| <3cm | |||
| >3cm | |||
| Early MMC instillation | 0.49 | 0.30-0.81 | 0.006 |
| Yes | |||
| No |
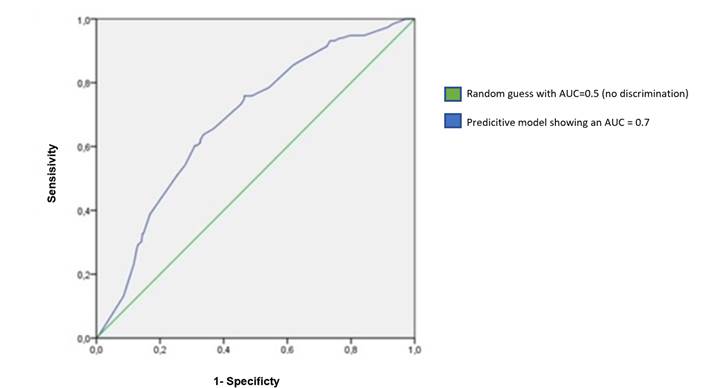
The predictive model shows an AUC=0.7 (IQ 0.62-0.73; p=0.0001) (Figure 1).
The formula that has generated our logistic regression model is:
Y |
is the dependent variable (tumor in second TURB= yes). |
X1, X2, X3, …,Xk |
are the independent variables identified |
Α, β1, β2, β3,…, βk |
are the parameters of the model (Coefficient B in the logistic regression). |
exp |
is the simplified exponential function. It corresponds to raising the number e to the power contained within the parentheses. The number e is Euler's constant base of natural logarithms whose value in thousandths is 2.718. |
In our case the variables of the equation are in column B of the logistic regression (Table 3).
Table 3 Variables in the equation
| B | E.T. | Wald | gl | Sig | OR | CI (95%) | |
| Primary tumor Stage (T1) | 0.235 | 0.339 | 0.480 | 1 | 0.488 | 0.126 | 0.65-2.46 |
| Primary tumor grade (HG) | 0.610 | 0.337 | 3.226 | 1 | 0.071 | 1.84 | 0.95-3.56 |
| Recurrence (yes) | 0.625 | 0.251 | 6.176 | 1 | 0.013 | 1.87 | 1.14-3.06 |
| Multifocality (yes) | 0.748 | 0.243 | 9.459 | 1 | 0.002 | 2.11 | 1.31-3.4 |
| Size (>3cm) | 0.402 | 0.267 | 2.266 | 1 | 0.132 | 1.49 | 0.88-2.52 |
| Early MMC instillation (yes) | 0.706 | 0.255 | 7.657 | 1 | 0.006 | 0.49 | 0.03-0.81 |
Logit (p) = -4.825 + 0.235x (primary=T1a) + 0.610x (high grader=yes) + 0.625x(recurrence=si) + -0.748x(multifocality=si) + 0,402x (size> 3,4) + -0.706(MMC=yes)
Probability of the tumor in the second TURB = Yes= 1/1 + e- logit(p)
Discussion
The objective of our study was the design of a predictive model of residual tumor, for optimizing the selection of patients who will receive a second TURB.
Residual tumor detection rates in the re-TURB was 28.6%, consistent with data described in the literature (ranging between 20%-60% of Ta and T1 tumors).2,3 The largest reviews on this topic were carried out by Cumberbatch et al. and Naselli et al. In the first one, Cumberbatch et al.2 carried out a systematic review including 31 publications analyzing re-TURBs in Ta and T1 tumors, but contrary to our study, including patients without presence of muscular tissue in the first surgery sample. They found residual tumor between 20-65% of cases, with detection rates four times higher in T1 tumors than in Ta. Our low residual tumor rate may be because incomplete resections were not included in the analysis. In the meta-analysis carried out by Naselli et al.3 they only analyzed T1 tumors and found that in the subgroup of patients with presence of muscle in the first TURB, residual tumor was detected in 47%, slightly higher than in our subgroup of similar characteristics (29.7%).
In addition, several studies have shown that there is a significant risk of upstaging after the first TURB in T1 disease, with upstaging to T2 on second TURB of up to 10%.8,9 In our study, upstaging to T2 was detected in 9 patients from the initial T1 subgroup (2.6% from the 349 T1 patients), so re-TURB was essential for the correct stratification and posterior treatment of these patients.
Similar results have been obtained in studies carried out in Latin America. In the study carried out by Chamlati-Cuello et al., analyzing the results of re-TURB in a population that included patients in stages Ta and T1, they obtained a residual tumor rate of 39% of the cases, and restaging to muscle-invasive in 2.3% of cases.10
Despite the high rates of recurrence, the findings during re-TURB on few occasions are clearly suspicious of tumor persistence or recurrence the most frequent finding is to objectify the scar of the previous resection or sfascellus, and in less than 5% a clear tumor lesion.11
Residual tumor detection rates and staging are associated with the quality of the initial TURB and the characteristics of the tumor.12 A TURB is considered of good quality when the resection is complete and including detrusor muscle, and the risk of detecting residual tumor can reach up to 75% if the resection was not optimal.12,13 Although in our study only patients with apparent complete resection and muscular detected in the sample of the first TURB were included, the resection was considered complete at the discretion of the surgeon, so this is another subjective factor depending of the staff performing the surgery.
Regarding tumour characteristics, in our series there was a higher rate of residual tumor detection in recurrent and multifocal tumors. High-grade tumors showed a trend towards statistical significance (p=0.07), although probably with a larger sample the result would be statistically significant. Both multifocal and high-grade are factors widely described in the literature as predictors of residual tumor detection.3,13 However, contrary to our results, recurrence does not seem to correlate as clearly with the rate of residual tumor detection in these and other similar studies.3,14
We also differ from similar studies in size tumor, which is a which stablished predicting factor.13,15 In our analysis residual tumor rates were higher in ≥3cm tumors (OR 1.49), but it was not significant (p=0.13). This could be due to the size of our serie, but it could also be because the size of the tumor is subjectively indicated by the surgeon during surgery.
There is little evidence on the effect of MMC on the results of the second TURB. In our study, patients who received early and single instillation with mitomycin had a lower detection rate in the re-TURB (OR 0.40; p=0.006). It has been shown that early instillations with mitomycin reduce the risk of early recurrence in intermediate-risk tumors, so by acting on residual tumor cells, it could reduce this rate. Divrik et al.16 compared the progression rates in two groups: one in which re-TURB and adjuvant mitomycin therapy was performed, and another in which only mitomycin was administered: progression rates were significantly lower in the first subgroup, suggesting that intravesical chemotherapy with mitomycin does not compensate for inadequate resection.
It is important to consider that MMC was administered according to the EORCT recommendations, according to the intrinsic characteristics of the tumor, in lesions with a lower risk of, so the lower residual tumor detection rate in our series may be due to tumor characteristics rather than mitomycin itself.
The second TURB therefore allows for further staging, thus helping to reduce recurrences and progression rates.2,9 However, some studies doubt the usefulness of re-TURB, affirming there is no the long-term benefits in terms of recurrence-free survival and progression-free survival, as they have not found differences in survival between high-grade T1 tumors treated with re-TURB plus BCG and those treated directly with BCG if muscle was present in the first intervention.17 Similar results were reported by Gontero et a.l, which only found advantages if muscle was not present in the original sample.14 In addition, it must considered that it is an invasive procedure, which requires general anesthesia, and is not free of complications, which entails a significant expense for the health system.5
In conclusion, it is necessary to adequately select the patients who can benefit the most from a second intervention based on the main predictive factors of residual tumor detection. Our predictive model shows a significant area under the curve of 0.7, thus showing an acceptable discrimination capacity, and could help select these patients.
Regarding to the limitations, this study is based on a retrospective design, which obviously makes it less reliable than randomized clinical trial (RCT). This is a single-institutional study, the surgeries were carried out over a prolonged period of time by multiple surgeons and the indication for early instillation of MMC was based on current scientific recommendations, but perhaps there is an added subjective criterion in your indication. Furthermore, the surgeon experience level can affect outcomes, and this was not documented in all cases. In addition, some prognostic pathological features are not uniformly reported, as for linfovascular invasion, sarcomatoid differentiation, nested tumors, etc. It is necessary to carry out multicentre, prospective, randomized studies assessing all factors to achieve strong conclusions.
Conclusion
In our study, recurrence and multifocality tumors are independent predictive factors for residual tumor in a second TURB, while high-grade shows a trend to statistical signification as risk factor and early administration of mitomycin-C behaved as an independent protector factor (probably be due to tumor characteristics rather than mitomycin itself).
Our predictive model evaluates the probability of finding residual tumor in re-TURB with a 70% accuracy. This way, we could optimize the selection of patients in low risk of residual tumor, thus avoiding unnecessary second surgery











 nueva página del texto (beta)
nueva página del texto (beta)