Background
A 50-year-old male who had no drug-allergy history was reported, but he did report a surgical history of cholecystectomy, and traumatic surgery of the right tibia-fibula. The patient didn´t report any clinical history or regular medication.
Clinical case
The patient had a previous consultation on 08/09 in another health center, showing symptoms of general malaise, myalgia, dry cough, anosmia, dysgeusia, dyspnea, and fever of 39°C, within a 4-day process. A nasopharyngeal swab was performed, and it was positive for SARS-CoV-2. On 08/12, he was transferred to the Intensive Care Unit, presenting HR 115 bpm, RR 24 bpm, BP 120/75 mmHg, temp. 36 degrees, and 90% on room air saturation with a requirement of oxygen therapy.
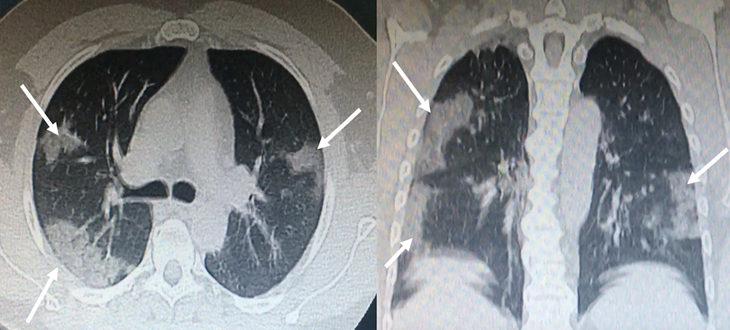
Ground glass opacities on chest computed tomography (CT) and bilateral infiltrates with a tendency for consolidation (Fig. 1). The patient signed an informed consent to receive plasma transfusion.
Upon admission, blood cultures x2 (HMC) and urine culture (UC), central line placement and right radial mean arterial pressure (TAM) were performed. Antibiotic treatment began with clarithromycin and ceftriaxone, dexamethasone and enoxaparin, 0.4cc every 12hs.
Figure 1: Bilateral, subpleural ground-glass opacities suggestive of SARS-CoV-2 pneumonia
Laboratory admission: RBC 39, Hemoglobin 13, WBC 15.5 (4.5 to 11.0 × 109 / L), Platelets 155, PT 95, APTT 35, BG 150, U 26, Creatinine 0.6, Ferritin 1361 (12-300 ng / mL), Fibrinogen 574 (200-400 ng / mL), D-Dimer 270 (up to 500 µg / L), Troponin <40 (up to 0.04 ng / mL), Na 140, K 3.6, Cl 103, CPK 267 (32-294 U / L), PCR +. Ca 1.12, Phosphate 2.4, Magnesium 2.4, TB 0.38, DB 0.15, ALP 92, GOT 47, GPT 143, Ac. Uric 2.4, LDH 685 (105-333 Ul / l). ABS: Ph 7.45, PCO2 37.9, PO2 74.6, HCO3 25, BE 1.6.
On 08/15, the patient reported lower right limb pain with variable temperature, a Doppler ultrasound was performed showing negative results for deep vein thrombosis.
On 08/19, the patient reported abdominal distension with decreased bowel sounds, diffusely painful on deep palpation, not peritoneal. Oral tolerance is suspended, and a nasogastric tube is placed without gagging or vomiting. An abdomen-pelvis CT scan is requested, informing the proximal/middle third of the right renal artery, with images of lack of filling in its interior, and hypodense areas in the right kidney, of triangular morphology with a peripheral base, that are visualized during the contrasted phase consistent with renal infarcts (Fig. 2-3).
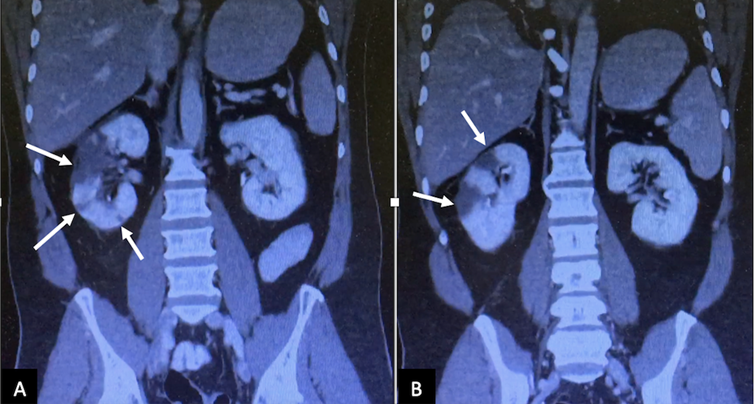
Coronal computed tomography image (A and B) in the nephrogenic phase showed multiple perfusion defects (arrows) in the right kidney, most pronounced in the upper pole, and lower pole too
Figure 2: Coronal computed tomography image
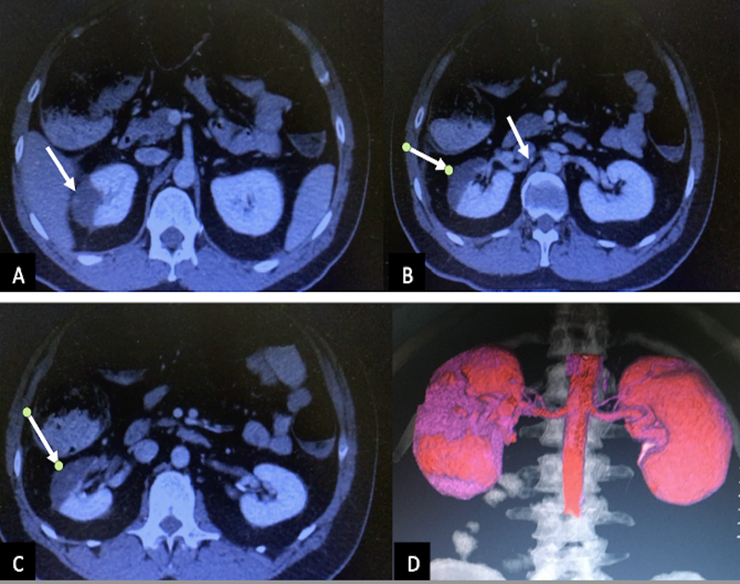
The axial abdominopelvic computed tomography image (A, B and C) revealed perfusion defects (arrows), which sharply demarcated a low attenuation lesion in the right kidney. There is visible thromboembolism in the renal artery (B, arrow). The coronal image (D) showed the areas without perfusion (violet color)
Figure 3: Axial abdominopelvic computed tomography image
On 08/24, an angiography was performed, with the presence of an ulcerated thrombus in the right renal artery, requiring percutaneous transluminal angioplasty (Fig. 4). It began with double antiplatelet therapy (ASA, acetylsalicylic acid and Clopidogrel) and continued with Enoxaparin.

The angiography revealed intramural hematoma of the right kidney artery, with occlusion (arrow). The percutaneous transluminal coronary angioplasty fixed the occlusion and improved the artery irrigation.
Figure 4: The angiography image
The patient improved favorably, without distension or abdominal pain, and good oral tolerance with spontaneous ventilation without oxygen requirement (97% saturation).
Due to clinical improvement, on 08/31 the patient was discharged from the hospital with anticoagulation for 6 months. A recent renal Doppler informed of renal vessels without stenosis. RD with slightly less vascularization and thickness in some cortical areas that appear sequential with respect to the contralateral one (Fig. 5). At present, the patient continues outpatient controls with hematology, cardiology and urology, with good renal function and significant improvement.
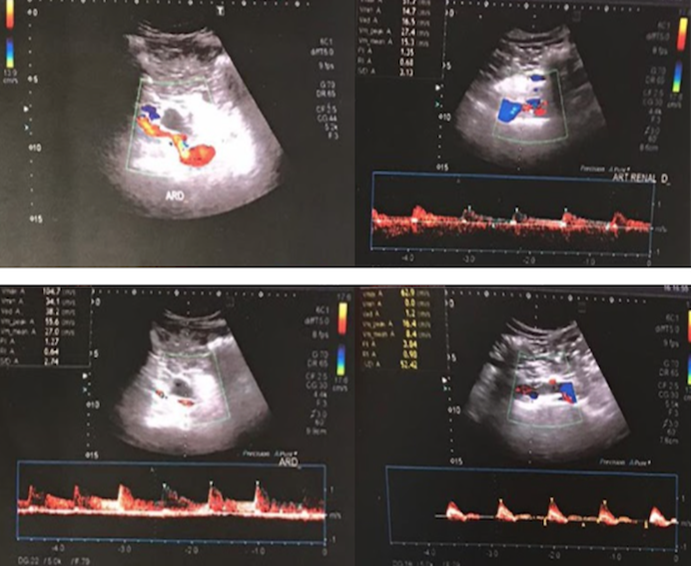
The renal Doppler informed of renal vessels without stenosis. The right kidney presented slightly less vascularization and thickness in some cortical areas. The angiography reported an ulcerated thrombus in the right renal artery.
Figure 5: The renal Doppler informed of renal vessels without stenosis
Discussion
The coronavirus disease-2019 (COVID-19) is a viral illness caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV2), and has been deemed a pandemic by the World Health Organization.1 SARS-CoV-2 is a single-strand RNA coronavirus, which enters human cells mainly by binding the angiotensin-converting enzyme 2 (ACE2), which is highly expressed in lung alveolar cells, cardiac myocytes, the vascular endothelium, and other cell receptors, like those of kidney and intestine.2 Angiotensin-II (AngII) is primarily metabolized by endothelial ACE-2 to the vasodilatory and anti-inflammatory peptide angiotensin. ACE-2 consumption by viral entry would be predicted to increase local AngII concentration. Among the known effects of AngII are vasoconstriction, endothelial activation, and pro-inflammatory cytokine release. Platelet activation by AngII may further enhance a prothrombotic milieu. AngII also has potent chemotactic effects that may accelerate lymphocyte recruitment and suppression.3
SARS-CoV-2 is transmitted primarily after viral particles are inhaled and enter the respiratory tract. In various series, hypertension, smoking, atrial fibrillation, obesity, peripheral vascular disease, previous thromboembolic events, diabetes and estro-progestin therapy are recognized as the main risk factors. Thus, patients with prior cardiovascular disease are at higher risk for adverse events from COVID-19.4
Initial symptoms overlap with other viral syndromes, and include fever, fatigue, headache, cough, shortness of breath, diarrhea, headaches, and myalgias. COVID-19 has the potential of resulting in severe illness, including systemic inflammatory response syndrome, acute respiratory disease syndrome, multiorgan involvement, and shock. Also, it may predispose hemostatic abnormalities in patients, including disseminated intravascular coagulation or arterial and venous thrombosis, including pulmonary emboli, cerebral infarcts, and limb ischemia. These thrombotic events in COVID-19 patients are higher. So, prophylactic anticoagulation reduces the risk of venous thromboembolism in these patients.5-9
Common laboratory abnormalities include lymphopenia, low levels of platelets and elevation in lactate dehydrogenase (LDH), and inflammatory markers such as C-reactive protein, D-dimer, ferritin, and interleukin-6. COVID-19 appears to induce a hypercoagulable state, with elevated fibrin/fibrinogen, minimal prolongation of prothrombin time, and activated partial thromboplastin time.10-12
Recent findings indicate an increased risk of acute kidney injury during COVID-19. The pathophysiological mechanisms which lead to acute kidney injury are not yet fully elucidated, but they may include direct cytopathic effects of the virus on kidney tubular and endothelial cells, indirect damage caused by virus-induced cytokine release, or kidney hypoperfusion due to a restrictive fluid strategy.13
The incidence of renal infarction is currently unknown. Renal infarction is usually due to thromboembolism with emboli arising from the heart or aorta in atrial fibrillation, although it can also be caused by other conditions. The clinical presentations are flank pain, nausea, vomiting, hematuria, proteinuria, and/or anuria, and mild fever, which are nonspecific. The rarity and non-specific clinical presentation of RI often lead to a delayed diagnosis. Renal angiography is the most sensitive test for diagnosis, but it is not performed on a routine basis. The improvements in CT technology plays an important role in the diagnosis of renal vascular disease. The laboratory tests performed included urinalysis and standard blood investigations (elevated LDH level). An earlier diagnosis may result in the preservation of renal function in more patients. There is controversy regarding the actual benefit of treatment after prolonged ischemia time.14,15
The treatment includes conservative therapy, such as hydration and blood pressure control, anticoagulation therapy (heparin infusion or a dose of warfarin), and intravascular therapy (thrombolysis and angioplasty). Some studies have reported successful revascularization after prolonged ischemic periods of up to 5 weeks. Several factors may affect the outcome of the treatment, such as ischemia time, collateral flow, and pre-existing kidney disease. The angioplasty is a safe modality of therapy and should be attempted for the purpose of kidney salvage, even in the setting of prolonged ischemia.16
Conclusions
COVID-19 can cause severe respiratory dysfunction combined with multiple complications including cardiovascular disorders, renal dysfunction, cerebral events and shock. Our patient did not present cardiovascular risk factors and presented the COVID-19 disease with oxygen requirement and renal infarction as a complication. Physicians should consider this problem, and renal artery dissection or renal artery intramural hematoma, in patients with abdominal pain without risk factors, when other intra-abdominal diseases are ruled out. There is currently no specific literature regarding the treatment of these patients. Perhaps, if this case had been treated with early angioplasty, it would have better kidney parenchyma.











 nueva página del texto (beta)
nueva página del texto (beta)



