Background
Kidney transplant surgery in Mexico has shown a constant growth since its beginning in 1963. In 2019, the Center of National Transplantation of Mexico reported that 2,967 kidney transplants were performed nationwide, of which 2041 (68%) were performed in living donors and 926 (31%) in cadaveric donors.1
Living donor nephrectomy (LDN) is a special procedure considering that it is performed in healthy individuals, so care must be taken to reduce morbidity to a minimum.1 Laparoscopic living donor nephrectomy (LLDN) has replaced the open approach, due to well-known advantages such as: better pain control, shorter recovery time, shorter length of hospital stay and better cosmesis.2 Moreover, we and other groups have demonstrated that functional results are comparable to those of open donor-nephrectomy.3 However, it has some drawbacks: longer warm ischemia time (WIT) and steeper learning curve.4
LLDN is a demanding procedure where vascular control is the most relevant step, in which surgeon must ensure an adequate length of renal artery and vein without compromising the donor and the graft, thus having to take action to minimize the potential of harm. There are two methods to secure the renal vessels laparoscopically: a transfixing method with staplers and a non-transfixing method with surgical clips, either of metal or self-holding polymer clips.5 The risk of malfunction exists with both devices: clip slippage or stapler misfire.6 In general, both situations are considered to be reported in the literature less frequently than actually occur.7 In 2006, the U.S. Food and Drug Administration (FDA) issued a recommendation against the use of polymer clips (Hem-o-lok®) for renal vessel control in LLDN, leaving as the only alternative staples to control renal vessels.8
Nevertheless, stapler misfire present in up to 1.7% of LLDN.9 Furthermore, the use of stapling devices represents higher costs for patients and healthcare systems in comparison polymer clips. For that reason, proving that the use of polymer clips is a safe and effective method for vascular control in LLDN could be a valid contribution for low- and mid-income healthcare systems or to reduce out-of-pocket expenses to patients.
The aim of our study was to describe the experience in our Tertiary-care center using polymer clips (Hem-o-lok®) for vascular control during LLDN.8
Materials and methods
We conducted a retrospective and descriptive analysis from a database of both hand-assisted and pure laparoscopic living donor nephrectomies performed from 2008 to 2020 in our institution by three urologists (BGP, JKB, FRC). Study protocol was approved by the Institutional Review Board (IRB:3408) and no research funding was needed. Surgical technique always comprised thorough dissection of fatty tissue surrounding renal vessels as well as placement of 2 polymer clips on the proximal end of the vessel before sharp transection, leaving a 2mm stump between clip and transection line to reduce the risk of clip displacement as Figure 1 A-D shows.
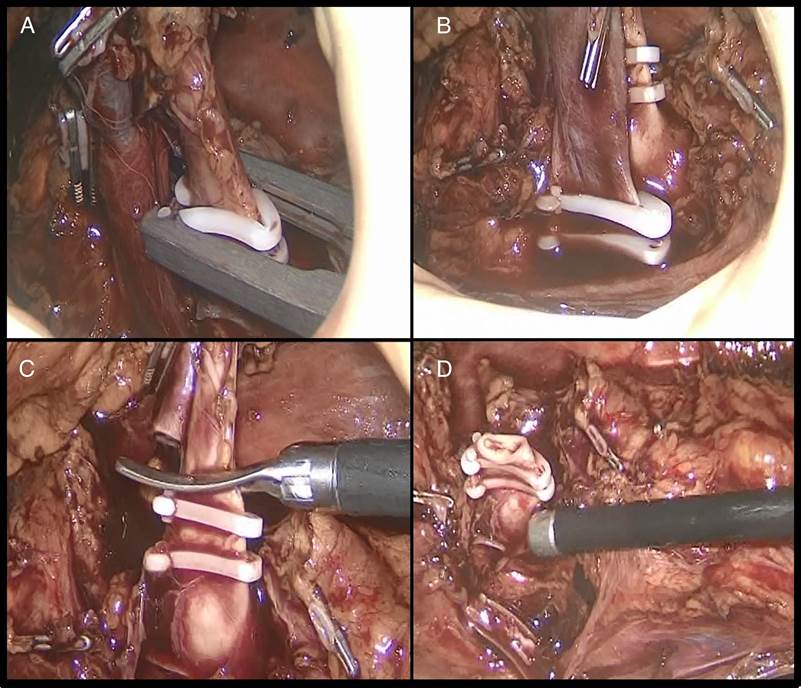
A) Circumferential dissection of renal vessels, thoroughly removing all surrounding fatty tissue as proximal as possible, with complete visualization of the clip applicator tip for ligation of renal artery; B) Placement of two clips proximally in both renal vessels (10mm clip on renal artery and 15mm clip on renal vein); C) Sharp transection of the vessel after confirming correct placement and D) Leaving a 2mm stump between clip and transection line to reduce the risk of clip displacement.
Figure 1A-D Example of the correct use of polymer clips in our center
Variables analyzed included age, gender, body mass index (BMI), preoperative and postoperative estimated glomerular filtration rate (eGFR), preoperative and postoperative hemoglobin and hematocrit, type of minimally invasive surgery (hand-assisted or pure laparoscopic), laterality, WIT, operative time, estimated blood loss (EBL), and need for blood transfusion. We also evaluated intraoperative and 30-day postoperative complications with the modified Clavien-Dindo Classification for LLDN.10 The descriptive analysis was performed using SPSS v 20.0 statistical software.
Results
A total of 380 LLDN were performed between 2008 and 2020 at our center. We excluded 50 patients due to incomplete information regarding vascular control device used or perioperative variables, leaving 330 LLDN suitable for analysis.
The median donor age was 39±18 years (range 18-69), with 187 women (56.7%). Mean BMI was 25.53 kg/m² (SD 2.95).
Preoperative data
Mean preoperative creatinine was 0.8 mg/dL (SD 0.17) with a median eGFR of 105±23 ml/min (57-137). The mean preoperative hemoglobin was 14.9 g/dL (SD 1.3), with a mean hematocrit of 43.84 (SD 4.06).
Intraoperative data
Two surgical techniques have been applied in LLDN throughout these twelve years at our center: at the beginning of our series 183 patients (55.5%) underwent hand assisted LLDN; subsequently, 147 patients (44.5%) underwent pure LLDN. The left kidney was retrieved more frequently (309 cases; 93.6%). Mean operative time was 235±61 minutes (range 120-156).
Mean WIT was 3.98 minutes (SD 2.022) and median EBL was 100±140 (range 10-2500). Four patients (1.2%) required conversion to open surgery due to vascular injury. Only in one case this was secondary to polymer clip displacement. The other three cases could be explained by excessive traction at the time of kidney retrieval. In all cases, the situation was solved with intraoperative repair and blood transfusion without further complications for donors or grafts. No reoperations, graft losses or donor deaths were reported in our series (Table 1). Kidney transplantation was completed successfully in all 330 cases.
Table 1 Summary of four patients requiring conversion to open surgery
| Patient | Gender | Age | BMI | Preop eGFR (ml/min) | Preop Hb (g/dL) | Side | EBL (ml) | Transfusion (Y/N) | Postop Hb (g/dL) | Postop eGFR (ml/min) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 33 | 26.9 | 132 | 13.6 | Left | 1200 | Y | 10.9 | 97 |
| 2 | Female | 23 | 25.5 | 102 | 14.8 | Left | 100 | N | 11.4 | 68 |
| 3 | Male | 34 | 23.5 | 100 | 15.7 | Left | 2500 | Y | 8.4 | 49 |
| 4 | Male | 30 | 24.2 | 110 | 14.9 | Left | 800 | N | 12.6 | 61 |
eGFR, estimated glomerular filtration rate; Hb, hemoglobin; EBL, estimated blood loss.
Postoperative outcomes
Thirty one complications (9.3%) were registered during the 30-day postoperative period, of which 23 (6.9%) were modified Clavien-Dindo grade 1 (Non-life-threatening complications); five (1.5%) were grade 2a complications (one pulmonary embolism, three pneumonias, and one intraabdominal abscess managed conservatively); there were only three (0.9%) grade 2b complications in two patients (one patient with a pancreatic fistula and an intraabdominal fluid collection requiring percutaneous drainage; and one patient with a pleural effusion and an intraabdominal abscess requiring thoracentesis and percutaneous drainage). Description is summarized in Table 2. No deaths nor loss of renal grafts were reported.
Table 2 Type of complications and number of patients. (*One patient presented 2 complications)
| Complications | Patients | Modified Clavien-Dindo |
|---|---|---|
| Gastrointestinal | ||
| Ileum and constipation | 3 | 1 |
| Melena | 1 | 1 |
| Respiratory | ||
| Pulmonary embolism | 1 | 2a |
| Pneumonia | 3 | 2a |
| Atelectasis and desaturation | 9 | 1 |
| Pleural effusion* | 1 | 2b |
| Genitourinary | ||
| Urinary tract infection | 3 | 1 |
| Epididymitis | 1 | 1 |
| Acute urinary retention | 3 | 1 |
| Surgical site infection | ||
| Seroma | 1 | 1 |
| Lymphatic fistula | 1 | 1 |
| Pancreatic fistula and intraabdominal fluid collection | 1 | 2b |
| Intraabdominal abscess* | 2 | 2a / 2b |
| Other Complications | ||
| Tramadol hypersensitivity | 1 | 1 |
| Intraoperative Complications | ||
| Conversion to open surgery | 4 | 2c |
Discussion
In any surgical procedure the goal is to perform it safely and with optimal results. For the case of living donor surgery these principles are of particular interest since it is performed in a healthy, usually young, altruistic donor. In addition, it imposes other technical difficulties such as ensuring an adequate vascular length to ease vascular anastomosis in the recipient, while reducing graft damage and ischemia. The most critical step of this surgery is a thorough dissection and control of renal vessels before transection. This can be achieved by using clips or staplers. Although both are effective methods for vascular control, failure has been reported. Nowadays, staplers are the only FDA approved method for vascular control of the renal hilum in the United States of America. However, the use of staplers is not free from complications, which have been reported to happen in 1-2% of the cases.11,12 In this study, we report 4 (1.2%) out of 330 patients having vascular complications in whom vascular control was achieved with polymer clips. This rate is similar to that estimated with the use of stapling devices. In fact, Hsi et al. analyzed the FDA database on the different methods for vascular control in LLDN estimating a complication rate of 1.7% with the use of polymer clips.8
One of the main reasons for polymer clip dislodgment or failure in securing blood vessels is the presence of excessive fatty tissue surrounding renal vessels, which blocks the self-locking mechanism of the clip.13 Therefore, a refined surgical technique which fulfills the following surgical principles is of paramount relevance: 1) thorough circumferential dissection of each vessel, completely removing all surrounding fatty/lymphatic tissue; 2) full visualization of the clip applicator tip and the self-locking end of the clip; 3) application of 2 clips on the proximal end of each renal vessel; 4) confirmation of the correct clip placement before securing it and transecting the vessel; 5) sharp vessel transection leaving a remnant of at least 2 millimeters between the clip and transection line; and 6) careful withdrawal of the clip applicator. Some studies have confirmed that, if these 6 steps for the correct placement of Hem-o-loks® are followed, the use of polymer clips can be considered a safe option for vascular control of the renal hilum.14,15
Regarding other intraoperative characteristics in our series, mean WIT, EBL and operative time are comparable to those reported by other groups using staplers, without clinical difference.16-18 Recently, a meta-analysis comparing the use of polymer clips and vascular staplers for donor nephrectomies found no differences in terms of mechanical failure rate, intraoperative bleeding or death confirming that the correct use of both devices is safe.19 Chan et al. retrospectively analyzed a series of 175 patients who underwent LLDN, with a complication rate of 14% (4% major complications), conversion rate of 1.7% and blood transfusion requirements of 3.4%.20 In comparison, our complication rate was 9.3%, most of which were non-life-threatening complications (23/31 cases), the remaining complications were subject to medical management or minimally invasive procedures (thoracocenthesis and percutaneous drainage). Similarly, we reported a low conversion rate and blood transfusion requirements of only 1.2%. There were no deaths nor loss of renal grafts reported among our patients.
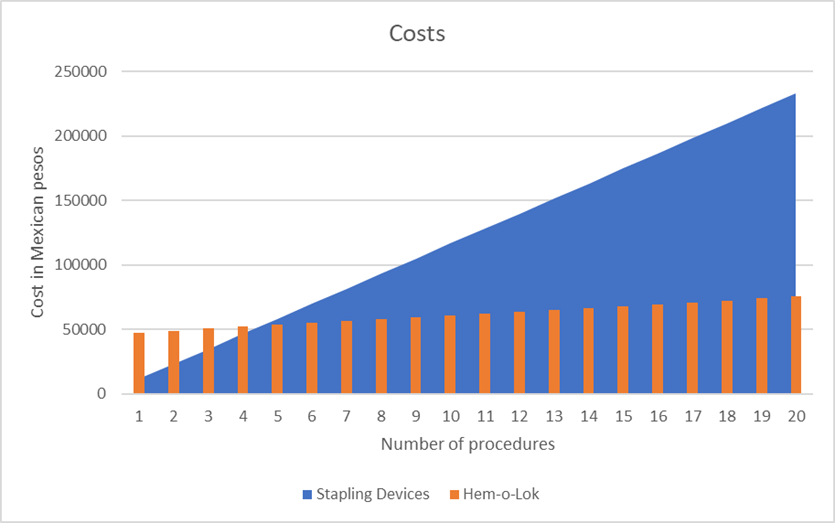
On the other hand, the question arises as to which option is more cost-effective and profitable in our setting. In Mexico, the cost per patient of a Covidienä endoGIA stapler with 2 vascular stapler loads is approximately $600 USD. In contrast, the cost per patient of a reusable clip applicator plus one clip cartridge is approximately $150 USD. Figure 2 shows the cost differences between using stapling devices compared to polymer clips after 20 procedures. Estimating a cost of $521 USD per patient in the stapler group results in a total expense of $10,000 USD after 20 procedures. Conversely, the estimated cost of the clip applicator is $2,000 USD, while the cost of 10mm (purple) and 15mm (gold) Hem-o-lok® cartridges is $21 and $40 USD, respectively, resulting in an approximate expense of $3,300 USD after 20 procedures. Therefore, when using polymer clips, we reach a cost-benefit advantage after 5-6 procedures.
Conclusions
According to our results, the use of self-locking polymer clips is a safe method for vascular control of the renal hilum vessels in LLDN. A careful dissection and refined surgical technique are essential to avoid perioperative complications. Additionally, this method appears to be the most cost-effective option, with similar outcomes when compared to stapling devices.











 nueva página del texto (beta)
nueva página del texto (beta)