Introduction
Sexual development in mammals is genetically determined. It is defined at a phenotypical level by the development of gender-specific anatomy, physiology, and behavior. At a cellular level, sex is defined by the chromosomal complement and the genetic orchestration.1,2 Sex-specific gonadal development starts with the formation of the bipotential gonad, which differentiates to testicular or ovarian tissue. The differentiation process depends on the activation of testis-specific or ovary-specific pathways, with parallel repression of the opposite pathway.3,4
The process for testis differentiation requires the participation of genes belonging to the SOX family. The SRY, located in the chromosome Y, was the first gene of the SOX family to be identified. This gene encodes a transcription factor sufficient and necessary to induce testis development.5 After its translation, the SRY protein translocates to the nucleus and interacts with the steroidogenic factor 1 (SF-1). The SRY protein and SF-1 bind to equal or similar places located within a testis-enhancer region in SOX9, inducing SOX9 expression.4-7 It has been determined that SF-1 (NR5A1 gene) regulates different developmental and functional aspects of the suprarenal gland and reproductive system. Activation of the expression of the AHM gene that encodes the Anti-Müllerian factor or hormone (AMH) is noticeable among those aspects.8 Activation of AMH is also regulated by the binding of WT1 transcription factor to the Anti-Müllerian hormone receptor 2 (AMHR2) promoter.9 The WT1 has been described as a transcription factor necessary for early gonad development. The WT1 and LHX9 together function as direct activators of SF-1.10 Another gene involved in sexual development is NR0B1(DAX1 protein). This gene, located on the X chromosome (p21.3), encodes an orphan nuclear receptor that functions as a transcriptional repressor for many other genes, including NR5A1 and some genes encoding steroidogenic enzymes.11,12
In ovarian differentiation, the participation of WNT4, RSPO1, and B-catenin (CTNNB1) is known.3,4 In XX gonads, WNT4 and RSPO1 signaling factors favor and stabilize the expression of the transcription factor CTNNB1 (known as B-catenin), which participates in SOX9 genetic repression. The WNT4 is widely known as a necessary factor for early ovarian development.13
Alterations in the testis-specific or ovarian-specific signaling pathways during gonadal development result in ‘disorders of sex development’ (DSD).3 The DSD are defined as congenital conditions with atypical gonadal or anatomic development of chromosomic sex.14
Collectively, DSD occur in fewer than one in 4,500-5,000 live births. The DSD include a clinical spectrum from hypospadias (1 in 200-300 births) to more severe conditions (exact prevalence unknown). Such is the case in 46,XY or 46,XX ovotesticular syndrome and 46,XY or 46,XX gonadal dysgenesis.4 The presence of internal and external female genitalia despite a 46,XY karyotype is the main characteristic of 46,XY complete gonadal dysgenesis, a DSD formerly known as XY sex reversal.15 Conversely, 46,XX gonadal dysgenesis is a primary ovarian failure that leads to premature ovarian insufficiency in otherwise normal 46,XX.16 The presence, histologically confirmed, of testicular and ovarian tissues in a case with 46,XX or 46,XY karyotype, defines the ovotesticular syndrome. The ovotesticular syndrome was previously known as true hermaphroditism.17 There is a hypothesis of an alteration in the signaling pathways involved in gonadal development as the cause for DSD,3 but the etiology of gonadal dysgenesis and ovotesticular syndrome in a majority of cases is unknown.18
Regarding XY gonadal dysgenesis, two types have been described: Complete (or pure) and partial. Partial gonadal dysgenesis is characterized by the presence of ambiguous genitalia, with or without Müllerian structures. Genes involved in partial gonadal dysgenesis include: SRY (by genic deletion or loss-of-function mutation);19,20NR5A1 (by of loss-of-function mutations); DHH (by loss-of-function mutation, homocygous or heterocygous, affecting Leydig’s cell differentiation); and MAP3K1 (heterocygous gain-of-function mutations, associated with increased β-catenine expression and SOX9 suppression).21-23 Also, in patients with 46,XY gonadal dysgenesis, partial Xp duplications including NR0B1 gene and 9p chromosomal deletions including DMRT1 and DMRT2 genes, have been described.21-24 Molecular mechanisms involving DMRT1 and DMRT2 leading to gonadal dysgenesis are not completely understood.24
Testicular syndrome, or 46,XX sex reversal, is caused by translocations involving SRY gen in 80% of cases.25 Regarding SRY-negative cases, copy number variations (CNV) affecting SOX3, SOX9, and NR5A1 genes, have been described.25-28 The SOX3 encodes a protein similar to SRY, sharing 90% of amino -acid identity in their DNA-binding HMG domain.29 Like SRY, such protein works synergistically with SF1 in the activation of the SOX9-enhancing region.30
For 46,XX ovotesticular syndrome, a small proportion of individuals with this condition have a fragment of the Y chromosome that includes the SRY gene, translocated to one of the X chromosomes. Likewise, CNV involving SOX9, SOX3, and NR0B1;18 and a recurrent variant (p.Arg92Gln) in heterozygous status in NR5A1 have been described.31 However, 46,XY ovotesticular syndrome is extremely rare and represents approximately 10-12.5% of ovotesticular syndrome cases. Pathogenic variants involving SRY, SOX9, DMRT1, and NR0B1 genes have been described as the cause.32
The objectives of this paper were two-fold. First, it characterizes the cytogenetic and molecular characterization of a group of patients with ovotesticular syndrome and complete gonadal dysgenesis from peripheral blood and gonadic tissue samples. Second, it analyzes the role of pro-testis- and pro-ovarian-pathways genes in the clinical phenotype.
Materials and Methods
Subjects of Research
This study was approved by the Ethics Committee of the Hospital Universitario San Ignacio, Bogotá D.C., Colombia (FM-CIE-0445-17). Informed consent was obtained from the subjects of research: 4 patients with 46,XX SRY(-) DSD (3 cases with diagnosis of ovotesticular syndrome and a case of suspected gonadal dysgenesis), and 2 patients with 46,XY SRY(+) DSD (a case with ovotesticular syndrome and a case with gonadal dysgenesis) (Fig. 1). All individuals were evaluated by the transdisciplinary joint committee of the Hospital San Ignacio, Bogotá D.C., Colombia. Genetic tests included karyotype, FISH for SRY, and MLPA from blood samples.33 In some cases, the tests were also performed in gonadic tissue. In this study, all selected patients were analyzed for CNV in DNA samples from peripheral blood. In cases where a biopsy was performed as part of the diagnostic approach, molecular tests were also performed from cultures of gonadic-tissue fragments (Fig. 1).

Figure 1 Summary of the studied cases with DSD and applied tests. Created with https://www.biorender.com/
Biological samples
Peripheral blood samples were used for karyotype, FISH, MLPA, and CGH. In some cases, gonadic biopsies were obtained (cases 1, 4, and 6). Gene-expression experiments used samples of culture of Sertoli cells isolated from human adult testicle (ScienCell#4520) as control tissue. In addition, cell line HS 1. Tes (ATCC CRL7002TM) was used. This cell line is composed of human testis cells of second-trimester embryo.
Cytogenetic Analysis (Karyotype and FISH)
Cytogenetic analysis was performed from lymphocyte culture obtained in peripheral blood and stimulated with phytohaemagglutinin (Gibco 10576015) following the protocol described by the research group.34
Gonad karyotype was performed from cultures of gonadic-tissue biopsies. The tissue was mechanically digested and cultured with RPMI medium and 20% SFB. Chromosomes prepared in slides were treated with HCl and Wright stain for G-banding. A total of 50 cells in metaphase were analyzed with a 550-bands resolution. Molecular cytogenetic analysis through Fluorescent In Situ Hybridisation (FISH) was performed with the use of a SRY-specific gene probe (SRY probe, Cytocell Aquarius). This reagent contains probes labelled in blue for the X chromosome centromere (DXZ1), a specific probe labelled in red for SRY gene (Yp11.31), and probes labelled in green fluorescence for Yq12 (DYZ1).
Extraction of genomic DNA
Extraction of DNA from peripheral blood and gonadic biopsy was performed using the Quick-DNATM Universal kit, following the manufacturer’s instructions. The concentration and purity of the DNA were evaluated by spectrometry with the NanoDropTM. The quality of DNA was assessed through electrophoresis in 1% agarose gel.
Multiplex ligation-dependent probe amplification (MLPA)
Possible deletions and duplications of SOX9, NR0B1, NR5A1, SRY, and WNT4 genes were studied with the MLPA SALSA P185-B2 Intersex kit (08 version; May 7, 2015) (MRC Holland), following the manufacturer´s instruction. After 35 PCR-amplification cycles, amplification products were separated with the genetic analyzer ABI 3100. Data were analyzed using the Coffalyser (MRC Holland®). Each sample underwent duplicate analysis. Peak areas for each probe were normalized to the average of peak areas in the three controls. Samples of DNA that showed a reduction or an increase in the values of the MLPA peak area were analyzed again using the same procedure.
Matrix-comparative genomic hybridization (CGH)
Analysis of CNV was performed with CGH using DNA from peripheral blood and culture of gonadic tissue, with a minimal DNA concentration of 66,592 ng/ul. The extracted DNA was sent to Macrogen Inc. for processing of the Array Chromosomal Study with the Comparative Genomic Hybridization (Array-CGH) technique on an Illumina Infinium OmniExpress-24 platform. A bioinformatics analysis was performed with the program GenomeStudio v2011.1 Genotyping 1.9.4, cnvPartition_v3.2.0. Genomic coordinates have been obtained from the reference human sequence NCBI37/hg19 (UCSC Genome Browser 2009).
Real time PCR (qPCR)
Messenger RNA levels for SRY, SOX9, and SOX3 genes were determined by q-PCR in gonadic tissue, Hs 1.Tes, and Sertoli cells. For all qPCR assays, RNA was isolated from tissue and/or cellular cultures with 100% confluence using the agent Trizol (Invitrogen®), following the manufacturer’s specifications. After RNA collection and quantification, the corresponding cDNA was synthetized. Once cDNA was procured, qPCR amplifications were performed using specific primers for SOX3, SOX9, and SRY (Table SI). For each 10 µl of q-PCR-TR reaction, 2.5 µl of DNA from the sample were added to a mixture containing 5 µl of SYBR Green (Invitrogen®), 0.5 µl of each primer in a 10 µM/ concentration, and 1.5 µl of ultrapure nuclease-free water. Results were analyzed using the Ct comparative method or 2-ΔΔCt as a strategy for relative quantification, with GAPDH as the reference gene. Gene expression results were graphically represented and analyzed with the software GraphPad Prim, version 6.
Immunofluorescence
Protein expression of SOX3, SOX9, and SRY was evaluated through indirect immunofluorescence in cultured cells from right and left gonads of a case with ovotesticular 46,XX SRY (-) DSD and in Sertoli cells as the positive control. On each slide, 100,000 cells from gonadic culture were seeded for immunofluorescence. Cells were fixed, washed, permeabilized, and incubated with primary antibodies directed against SRY (Anti-SRY antibody [OT13C8] ab140309) 1:100; SOX9 (Anti-SOX9 ab3697) 1:100; and SOX3 (Anti-H-SOX3 AF2569 R&D systems). Secondary antibodies were Alexa Fluor 594 Donkey Anti-Goat IgG (orange fluorochrome), Alexa Fluor 488 Invitrogen Goat Anti-Mouse IgG (green fluorochrome), and Alexa Fluor 647 Invitrogen Goat Anti-Rabbit IgG (red fluorochrome). Finally, slide mounting was performed with 7 ul of mounting medium ProLongTM Gold Antifade Mountant with DAPI (Invitrogen, P36931) for staining of contrast nuclei. Images with a 640 x 640 pixels resolution were obtained with a laser scan confocal microscope FV1000 (Olympus, Tokyo, Japan) using the oil immersion objective with UPLSAPO 60 x 1.35 NA. Images were processed using the software Image J.
Results
Six patients with DSD were included: 3 with diagnosis of 46,XX SRY(-) ovotesticular syndrome, a patient with diagnosis of 46,XY SRY(+) ovotesticular syndrome, a patient with suspected 46,XX SRY(-) gonadal dysgenesis, and a patient with complete 46,XY SRY(+) gonadal dysgenesis (Table I).
Table I Summary of the main histological and cytogenetic findings of the studied cases.
| DSD Type | Case | Assigned sex | External phenotype | Internal phenotype | Gonadal biopsy | Gonadal karyotype | Peripheral blood karyotype |
|---|---|---|---|---|---|---|---|
| 46,XX SRY(-) Ovotesticular | 1 | M | 1.8 cm genital tubercle, distal penis urinary meatus, palpable gonads, and urogenital sinus | No deferent duct, but a fibrous remnant with origin at the deep inguinal ring, crossing the midline to merge with the contralateral was found | RG: ovarian tissue with stroma and ovocites LG: ovarian tissue with stroma and ovocites. Immature tubules with Sertoli cells. Fragment of testicular and ovarian parenchyma | mos 92,XXXX[8]/46,XX[42] | 46,XX |
| 2 | M | Genitalia with hyperpigmented labioscrotal folds, without palpable gonads, 1 cm-long phallus, urinary meatus at the base of the phallus with mixed introitus | Uterus and gonads | RG: testis differentiation LG: testis and ovarian differentiation | Not available | 46,XX | |
| 3 | M | 3 cm genital tubercle, 1.3 cm-long urethral plate, hypospadiac meatus in perineal region, mild penoscrotal transposition | Spermatic cord, deferent duct, epididymus dissociated from the gonad | Incisional biopsy of gonad with reddish and velvety appearance shows seminiferous tubules, compatible with testicular tissue. Incisional biopsy of yellowish-brown gonad shows Graff follicles, compatible with ovarian tissue. | Not available | 46,XX | |
| 46,XY SRY(+) Ovotesticular | 4 | F | 7.5 cm x 2.5 cm genital tubercle, labioscrotal folds, urethral orifice, vaginal introitus orifice, 1.5 cm perineal body | Rudimentary uterus. Right uterine tube ending in proximity with righ, 2.5 cm, and intra-abdominal gonad. Left uterine tube more hypoplasic, ending in 0.5 cm irregular and soft gonad | RG: testicular tissue and ovarian stroma without follicles LG: uterine tube, deferent duct and epididymus | 46,XY SRY(+) | 46,XY |
| Suspected 46,XX SRY(-) gonadal dysgenesis | 5 | M | 3 cm genital tubercle, Prader 4, bilateral palpable gonads | Uterus and intra-abdominal gonads | Right and left ovaries negative for malignancy. Left ovary with follicular cysts | Not available | 46,XX |
| 46,XY SRY(+) gonadal dysgenesis | 6 | F | External genitalia with hypotrophic labia majora and labia minora; normal clitoral hood and clítoris, 1.5 cm genital introitus | Right uterine tube. Gonads located directly above the internal inguinal ring. Epididymis, and a deferent duct | RG/LG: streak gonad. Müllerian remnants, uterine tube. | 46,XY SRY(+) | 46,XY |
RG: right gonad, LG: left gonad
Cases with ovotesticular syndrome 46,XX SRY(-) diagnosis
Case 1
Case 1 was an individual with ambiguous genitalia, assigned male. Physical examination during neonatal period showed a 1.8 cm genital tubercle, distal penis urinary meatus, palpable gonads, and urogenital sinus. The patient had peripheral blood karyotype 46,XX; negative FISH for SRY (Fig. 2 A and B), negative MLPA Intersex, and negative aCGH for CNV associated with ovotesticular syndrome. The results of conventional cytogenetic, FISH and MLPA assays were included in a previous work of our research group.33
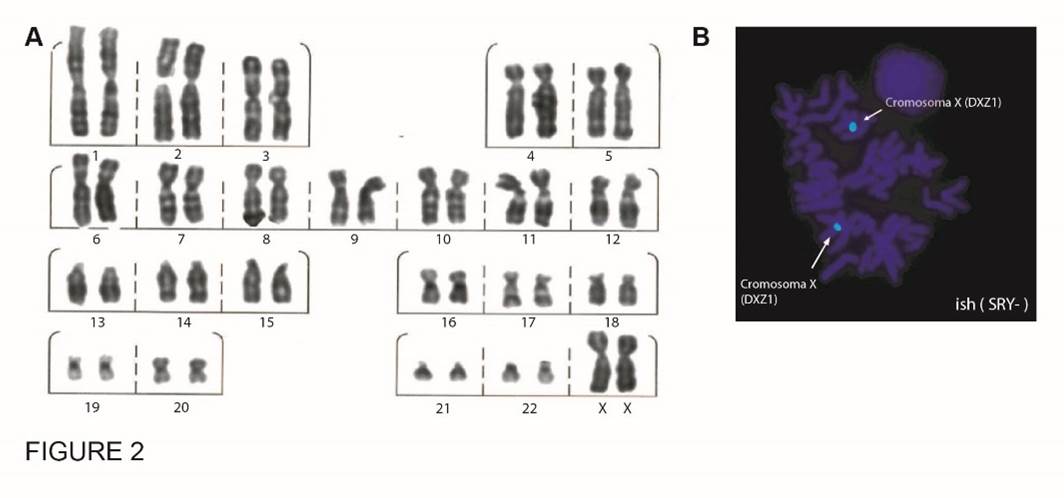
Figure 2 Cytogenetic analysis case 1. A. Karyotype by G-banding in peripheral blood, 46,XX. B. SRY FISH in peripheral blood
The image shows the metaphase of a 46,XX SRY (-) cell obtained with Cytocell detection kit that uses three fluorescent probes. X chromosome centromere region (DXZ1) labelled blue, SRY gene (Yp11.31) labelled red, and Yq12 (DYZ1) labelled with green fluorescence. C. Karyotype by G-banding in gonadal tissue in case 1,33 that showed chromosomal mosaicism with 2 cell lines: 92,XXXX[8]/46,XX[42]. The results of conventional cytogenetic, FISH and MLPA assays were included in a previous work of our research group
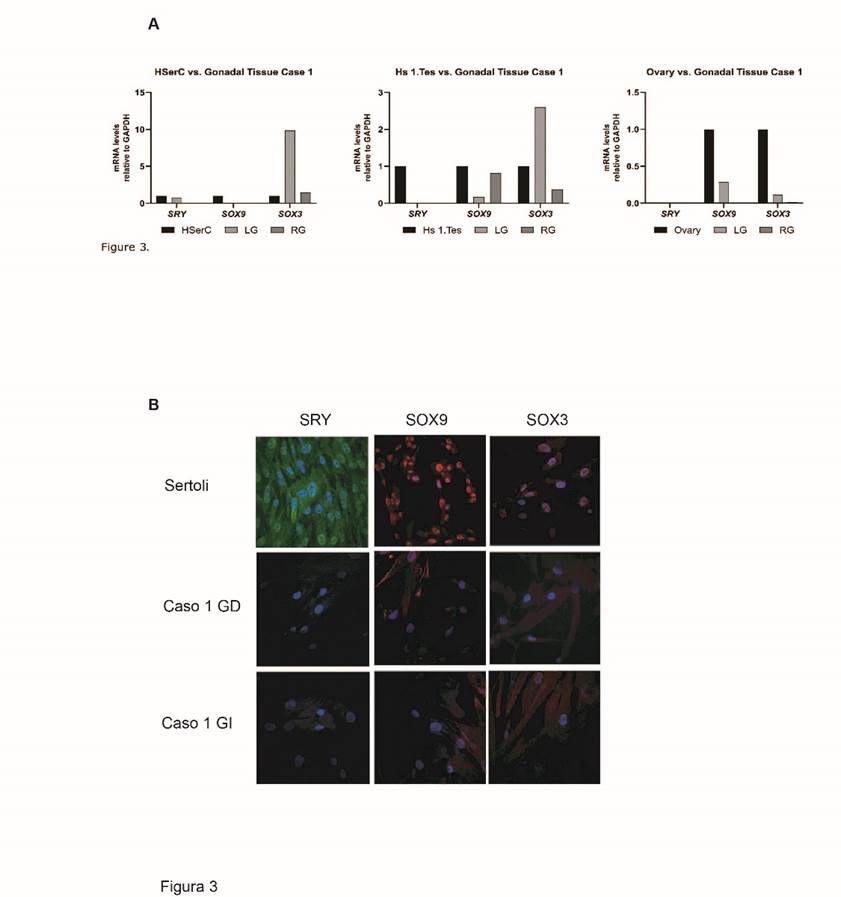
Levels of mRNA for SRY, SOX9, and SOX3 in gonadal tissue were determined by qPCR. Results from right and left gonads showed that expression levels of SRY and SOX9 mRNA were lower than the levels detected in Sertoli cells and embryonic human testis cells. Regarding SOX3, an increased expression was found in the left gonad (Fig. 3 A). Such expression levels of SRY and SOX9 in both gonads were consistent with low protein levels detected in immunofluorescence assays (Fig. 3 B). Regarding SOX3, protein expression was found only in the left gonad, which correlates with findings for mRNA (Fig. 3 B).
A. Gene expression of SOX3, SOX9 and SRY mRNA relative to GAPDH in right and left gonads of the case with 46,XX SRY(-) ovotesticular syndrome (case 1). B. Inmunofluoresence for SOX3, SOX9 and SRY in right and left gonads in case 1 and Sertoli cell as control. Alexa fluor 594 Donkey Anti-Goat IgG used as secondary antibody for SOX3 (orange fluorochrome), Alexa fluor 488 Invitrogen Goat Anti-Mouse IgG for SRY (green fluorochrome), Alexa fluor 647 Invitrogen Goat Anti-rabbit IGG for SOX9 (red fluorochrome). Nuclei are observed stained in blue.
Case 2
Case 2 was an individual with ambiguous genitalia, assigned male. The patient had karyotype with 46,XX chromosomic complement, SRY-negative FISH, and negative MLPA. No CNV were detected with aCGH of DNA from peripheral blood. There are no gonadal cytogenetic and/or molecular results for this patient, as no gonadal tissue was available for the study.
Case 3
Case 3 was an individual assigned male, with evidence of hypospadias and bilateral cryptorchidism. Laparoscopy showed gonads of mixed aspect. One gonad had a reddish and velvety appearance, and the incisional biopsy showed seminiferous tubules, compatible with testicular tissue. The other gonad was yellowish-brown and the incisional biopsy showed Graff follicles, compatible with ovarian tissue. The patient had peripheral blood 46,XX karyotype, SRY-negative FISH, and negative MLPA Intersex. No pathogenic CNV were detected with aCGH of DNA from peripheral blood. There are no gonadal cytogenetic and/or molecular results for this patient, as no gonadal tissue was available for the study.
Case diagnosed with 46,XY SRY(+) ovotesticular syndrome
Case 4
Case 4 was an individual assigned female who underwent laparoscopy at age 12 due to primary amenorrhea. Histological examination of the left gonad biopsy showed uterine tube fragment, deferent duct fragment, and epididymal fragments. Histological examination in the right gonad showed evidence of testis with atrophic changes and an area suggestive of ovarian stroma. A new gonadal biopsy performed 3 years later showed gonadoblastoma in the right gonad. The patient had peripheral blood 46,XY.ish Yp11.31(SRY+) karyotype, negative MLPA Intersex, and negative aCGH for pathogenic CNV. Gonadal karyotype, FISH, and MLPA results were consistent with those in peripheral blood. The aCGH from gonadal tissue did not detect CNV associated with ovotesticular syndrome. Examination of genomic DNA from the right gonad showed a heterozygous loss of the arr[hg37]:2q14.2(121746975-121747688)x1 region involving the GLI2 gene.
For mRNA expression levels of SRY, SOX9, and SOX3, in the right gonad SRY levels were similar to levels in Sertoli cells and lower than levels in the Hs1.Test.Relative SOX9 mRNA levels showed a differential expression in each gonad: The left gonad exhibited lower expression levels, while the right gonad showed nearly a five-fold increase compared to the control. The SOX3 mRNA expression showed an increase only in the left gonad (Fig. 4).
Case with suspected 46,XX SRY(-) gonadal dysgenesis
Case 5
Case 5 was an individual assigned male. Biopsy of right and left gonads showed ovarian tissue, the left with follicular cysts. The patient had peripheral blood 46,XX karyotype, SRY-negative FISH, negative MLPA Intersex, negative SRY sequencing, and aCGH of DNA from peripheral blood negative for CNV. There are no gonadal cytogenetic and/or molecular results in this patient, as no gonadal tissue was available for the study.
Case with 46,XY SRY(+) gonadal dysgenesis
Case 6
Case 6 was an individual, assigned female, in study for amenorrhea at age 12 without evidence of the uterus in imaging studies. Diagnostic laparoscopy showed two gonads located directly above the internal inguinal ring, epididymis, and a deferent duct. No Müllerian remnants were found. Gonadal biopsy reported streak gonad, Müllerian remnants, and right uterine tube. The patient had peripheral blood 46,XY.ish Yp11.31(SRY+) karyotype, negative MLPA for NR0B1, NR5A1, SOX9, SRY, WNT4 deletion/duplication, and aCGH negative for CNV associated with gonadal dysgenesis. Results of karyotype, FISH, MLPA, and aCGH in gonadal tissue were consistent with those in peripheral blood.
For SRY, SOX9 y SOX3 mRNA levels, the right gonad had SRY levels similar to levels in Sertoli cells and lower than levels in Hs1.Test. Relative SOX9 mRNA levels were lower than levels in Sertoli cells and higher than levels in Hs1.Test. The SOX3 expression was higher compared to Sertoli cells and Hs1. Test.
In the left gonad, SRY levels were similar to levels in Sertoli cells and lower than levels in Hs1.Test, while SOX9 levels were lower compared to Sertoli cells and Hs1.Test. For SOX3, higher relative expression levels were observed compared to Sertoli cells, and similar levels to those observed in Hs1.Test (Fig. 5).
Discussion
This paper presents three patients with 46,XX ovotesticular syndrome, 1 with 46,XY ovotesticular syndrome, 1 with suspected 46,XX gonadal dysgenesis, and 1 with 46,XY complete gonadal dysgenesis. In the three patients, chromosomal rearrangements involving SRY gene were ruled out during the initial diagnostic approach. Unexplainable cases of ovotesticular syndrome or gonadal dysgenesis can emerge from pathogenic variants in the pro-ovarian or pro-testis pathways. The CNV are detected in approximately 21.5% of DSD cases.35 For this reason, after ruling out deletions or duplications in the main genes (NR5A1, NR0B1, SOX9, SRY, and WNT4) of such pathways, a possible CNV involved in the patient’s phenotype was assessed through aCGH. No CNV were detected, however, that could explain the DSD of at least one of the cases presented, which falls into the expectations due to the test diagnostic performance.
In cases with access to gonadal tissue, cytogenetic and molecular characteristics were analyzed through karyotype, aCGH, and genic expression levels for SRY, SOX9, and SOX3.3,36 Study of SOX3 was considered, given the synergistic function of SOX3 with SF1 in the activation of SOX9 enhancer region and due to evidence of its etiological role for XX male (sex reversal) with genomic rearrangements involving SOX3 regulatory region.26,27,30
In case 1, with 46,XX ovotesticular syndrome, SOX3 expression levels in gonads were higher than levels in Sertoli and Hs1.Tes. Such findings might relate to the mosaicism found in gonadic tissue, mos 92,XXXX[8]/46,XX[42], given that tetraploidy is associated with chromosomal unbalance in gene expression.37 This unbalance would explain the level of SOX3 expression observed. The difference between karyotype in peripheral blood and karyotype in gonadal tissue is remarkable, however, given that chromosomal mosaicisms or chimerism in gonads have been previously observed, even with the presence of cells containing Y chromosome only in gonads.38 The above is supported by the hypothesis that in DSD unexplainable by studies in peripheral blood, gonadal mosaicism might explain the clinical phenotype of some patients, which justifies studies in gonadal biopsy.
In case 4 with 46,XY ovotesticular syndrome, gonadal SOX3 expression levels were higher compared to normal human Sertoli cells and Hs 1.Tes. Also, gonadal SRY and SOX9 expression levels were lower than levels in Hs 1.Test and Sertoli, respectively. Such a finding might explain the presence of ovaric stroma. Also, study of CNV for the right gonad showed a heterozygous deletion in 2q14.2 involving the GLI2 gene (glioma-associated oncogene 2 for the zinc finger protein). This encodes a transcription factor that participates in the Hedgehog signaling pathway. The Hedgehog pathway is involved in determining cellular destination and proliferation, and organ pattern during embryo development.39-41) Differentiation of fetal testis and postnatal and adult ovaries is especially important among such functions.40,41 Expression of Gli2 together with Gli1 has been observed in fetal Leydig cells. The Gli2 and Gli1 might play redundant functions in the differentiation process.40 Physical and functional interaction of GLI1 and GLI2 has recently been described in humans as regulating the expression of target genes, such as BCL2, MYCN, PTCH2, IL7, and CCD1 in pancreatic tumor cells PANC1.42 It is still to be determined whether those genes play a similar role in the human Sertoli cell-differentiation process. If so, the involvement of one of those genes might impact the right male sexual differentiation.
In case 5, with suspected 46,XX SRY(-) gonadal dysgenesis and assigned sex male, cytogenetic and molecular studies in peripheral blood do not elucidate the clinical condition’s origin. It is remarkable that the biopsy of gonadal tissue reports a right ovary and a left ovary with follicular cysts, especially as hormonal studies ruled out hyperandrogenism that might explain the virilization (data not shown). Evidently, there is not a confirmed diagnosis as of this date. Definition of 46,XX SRY (-) gonadal dysgenesis as a primary ovarian failure that leads to premature ovarian insufficiency in otherwise normal 46,XX females.16 For these reasons, the study presents this as a possible case of gonadal dysgenesis with atypical presentation. Although the underlying cause is unknown in most cases, alterations have been identified in some of the genes involved, including homozigous or compound heterozigous inactivating mutations in the gene of the follicle-stimulating hormone receptor (FSHR), mutations in the BMP15 gene, and mutations in the NR5A1 gene. Inactivating FSHR mutations are inherited in a recessive autosomal pattern; BMP15 mutations are X-linked inherited; and NR5A1 are dominant autosomal, in the majority of cases.43,44 In another connection, study of the expression of some genes in gonads of 46,XX SRY(-) patients with a masculine gonadal phenotype suggests that these types of conditions can be explained in some cases by alterations in gene expression. Kojima et al. in 2008, compared SOX9, NR0B1, Ad4BP / NR5A1, WT-1, GATA-4 and AMH expression in testis tissue of four 46,XX SRY (-) patients, with XY y XX normal controls. Their study found SOX9 expression levels in testis of these patients 1.9 times higher than levels in XY normal testis, while Ad4BP / NR5A1, NR0B1 y HAM expression levels were lower compared with the normal XY control.45 Also, copy number alterations were studied for these genes, although changes were not found at that level. Such findings indicate that SOX9 overexpression is critical in sex determination in 46,XX SRY(-) males, that reduced expression of genes as Ad4BP/ NR5A1, NR0B1, and HAM might also contribute to a masculine phenotype in these patients,44 and that alterations in expression of genes involved in sexual differentiation are not necessarily related to CNV. This suggests it is biologically plausible that epigenetic regulatory mechanisms are related to sex determination and gonadogenesis.
In case 6 with 46,XY SRY(+) gonadal dysgenesis, SOX9 expression patterns are conspicuous. Results for SRY expression in both gonads were similar to expression in Sertoli cells, however, SOX9 expression was very low compared to the control. Such low expression levels might explain a failure of the male differentiation process despite the presence of SRY. For sexual determination of most mammals, Sry gene expression induces the embrionary bipotential gonad to a testis destination via pre-Sertoli cell differentiation. These cells are essential, in fact, for testis differentiation. Expression pattern for Sry is strictly time/space controlled, and it is limited to Sertoli cells precursors. In mice, approximately 4 hours after the start of Sry expression, SOX9 is upregulated in Sertoli cell precursors. Analysis in the bipotential gonad has shown that Sry or Sox9 expression suffices to induce male development.45,46 For all these reasons, the authors highlight the limitation of gene expression assessment with a precise time determination during the embryonic period. The authors consider this paper an approach to gathering knowledge about gene expression and its important role in gonad differentiation in patients with DSD.
Conversely, no direct relationship was found between the SOX3 gene and DSD in the cases of this study. The findings, however, lead to the conclusion that the SOX3 gene expresses in non-differentiated gonadal tissue. In some cases, it might be involved in the clinical phenotype of patients with DSD. This shows that SOX3 overexpression is, as previously discussed, fundamental for intervention in the sexual determination pathway.30
It is also important to emphasize that malignancy is associated with DSD, mainly with the 46,XY DSD.47 There’s an estimated risk of gonadoblastoma of 15-35% for gonadal dysgenesis and 3% for ovotesticular syndrome. In this study, the patient in case 4 with 46,XY SRY(+) ovotesticular syndrome developed a gonadoblastoma detected incidentally during diagnostic laparoscopy that took a biopsy of the right gonad. Neoplastic transformation has been associated with deregulation of the tandem repetition region of TSPY, an acknowledged tumor-suppressor gene located inside the Y chromosome gonadoblastoma locus (GBY).48,49 The gene plays normal roles in masculine germinal cells proliferation and differentiation, but it expresses ectopically in early and late stages of gonadoblastomas, in situ testicular carcinoma (the pre-malignant precusor for all testicular germinal-cells tumors), seminoma and non-seminoma. Aberrant expression of TSPY induces stimulation of protein synthesis, acceleration of cell proliferation, and tumorigenicity.48
It is worth noting that, currently, fewer than 20% of patients with DSD receive a precise genetic diagnosis which justifies a continued exploration of new pathways and genes involved in the process of sex differentiation.50 This will allow further understanding of the underlying molecular mechanisms for human sexual development pathology including, for instance, diagnostic cytogenetic and molecular study in gonads as a complement to studies in peripheral blood. Such studies would be a potential source for understanding the clinical conditions of patients with DSD that are XX or XY in peripheral blood.
Conclusion
This study shows that, in some cases, cytogenetic and molecular study in peripheral blood are insufficient for diagnosing patients with DSD. A complementary approach, including gonadal cytogenetic and molecular analysis, can potentially improve diagnosis and enrich understanding of clinical manifestations with DSD that are XX or XY in peripheral blood.











 nueva página del texto (beta)
nueva página del texto (beta)