Introduction
Tuberose (Polianthes tuberosa L.) is a tuberous, herbaceous and perennial plant commonly known in Mexico as nardo, amole, tuberosa blanca, amiga de noche, Azucena and vara de San José (González-Vega, 2016). It is an ornamental plant native to Mexico that was dispersed around the world in the 16th century (Barba-González et al., 2012). Tuberose is a species that occupies an important position among ornamental bulbs because it is commercially used as a cut flower and ornamental potted plant, and essential oils are extracted from the flowers, which are highly valued (Kameswari et al., 2014). In Mexico, Cuba, India, New Zealand, and Japan, the tuberose is used as a cut flower in social events and for floral arrangements due to its appreciated aroma (Barba-González et al., 2012; González-Vega, 2016).
In Mexico, especially in Morelos, Oaxaca, Guerrero, Veracruz, and Puebla, 306.1 ha of tuberose are cultivated as cut flowers, generating 61.9 million pesos in annual sales (Servicio de Información Agroalimentaria y Pesquera [SIAP], 2020). In Morelos, tuberose is among the main cut flower crops (SIAP, 2020), where the variety with single flowers is ‘Mexicano’, and the variety with double flowers is ‘Perla’, which is grown in greater proportion (Vázquez-García, 2004). Internationally, there are varieties of tuberose such as ‘Doble excelsior’, ‘Tall double’, ‘Florentiu’ and ‘Orange flower’ (González-Vega, 2016). India reports varieties such as ‘Shiringar’, ‘Vaibhav’, ‘Calcuta doble’ and ‘Prajwal’, in which their behavior in cultivation and postharvest has been evaluated (Pérez-Arias et al., 2019).
Cut flowers, immediately after harvesting and before storage, or after storage and subsequent transport (but before applying vase or preservative solutions), are placed in a) rehydration solutions, which aim to restore turgidity (generally with water at room temperature, which contains some germicide, wetting agent and acidifier, without sugar); b) pulse solutions, which consist of placing, for a short period (from seconds to hours), the recently harvested flowers in a solution containing silver salts or acidifier, a germicide and high amounts of sugar; and c) solutions for opening flower buds, which are used when flowers are cut in bud stage and contain moderate amounts of sugar and a germicide (Arief-Zargar, 2016; Reid, 2009).
The use of preservative solutions is the most economical and practical method to extend the post-harvest life of cut flowers; in addition, they help preserve a fresh appearance for longer, so florists or consumers use them to keep flowers in saleable condition or vase life for a longer period. These solutions usually contain a germicide, sugars, pH regulator and sometimes surfactants and hormones (Kumar et al., 2017; Nowak & Rudnicki, 1990; Salunkhe et al., 1990).
The tuberose is a cut flower with a short postharvest life, which varies between 4 and 13 d (Pérez-Arias et al., 2019). Once harvested, respiration and ethylene production increases, requiring hydration and sugar supply to obtain a greater opening of lowers and increase shelf life (Kumari et al., 2018; Perez-Arias et al., 2019). Wahitaka et al. (2001) indicate that harvesting tuberose flower spikes with two or three open basal flowers, pulse pre-treatment with 20 % sucrose, and storage at 0-5 °C can maintain flower spike quality longer.
In the case of the tuberose, several authors point out that preservative solutions with sucrose (Sac) + citric acid (CA) or CA only increase the life of the tuberose from 1 to 10 d, if compared to flowers that were only placed in water (Sao & Verma, 2020; Sigma et al., 2018). Anjum et al. (2001) report that ascorbic acid (AAsc) in doses of 200 mg·L-1 increased the post-harvest life of tuberose by 1 d compared to control flowers. In commercial form, there is a product called FloraLife® Crystal® (which contains food for cut flowers), which precipitates dust and dirt particles, and keeps the vase water clean and odorless during flower opening and growth, this by applying a dose of 5 g·L-1 (FloraLife, 2020).
In preservative solutions, the germicide 8-HQC is the most used to control bacteria, yeasts and fungi (Kumar et al., 2017). Sucrose at 1 or 2 % is the most used source of energy to provide energy to flowers; acids or salts added to adjust the pH (between 3.5 and 5.0) are necessary to reduce the development of some microorganisms and to make water consumption by flower stems easier (Kumar et al., 2017).
Therefore, the aim of this study was to evaluate the application of preservative solutions (Crystal®, sucrose [Sac] + citric acid [CA] + hydroxyquinoline citrate [HQC] or ascorbic acid [AAsc]) on some physical, physiological and chemical changes of flower spikes of varieties ‘Mexicano’ and ‘Perla’ during postharvest.
Materials and methods
Plant material
Tuberose flower spikes from the varieties ‘Mexicano’ and ‘Perla’ were harvested from commercial orchards located in Cuahuchichinola, Mazatepec, Morelos (18° 38’ 54’’ N and 99° 22’ 56’’ W, at 996 masl). The harvest was carried out at 8:00 a.m., and the tuberose flower spikes were moved to the Agricultural Production Laboratory in the Faculty of Agricultural Sciences of the Universidad Autónoma del Estado de Morelos, where those that did not have pathogen damage and had at least two open basal flowers, were selected. Approximately 100 tuberose flower spikes of each variety were cut at 40 cm, and in each group were formed two lots of 50 inflorescences, which were placed under four preservative solutions (Table 1).
Table 1 Preservative solutions evaluated on the post-harvest life of ‘Mexicano’ and ‘Perla’ tuberose.
| Product | Concentration | pH | Electrical conductivity (μS·cm-1) |
|---|---|---|---|
| Crystal® | 10 g·L-1 | 3.45 | 387 |
| Sac + CA + HQC | 2 % +100 mg·L-1 + 100 mg·L-1 | 3.63 | 183 |
| AAsc | 200 mg·L-1 | 3.54 | 133 |
| Water | Control | 5.3 | 8 |
Sac = sucrose; CA = citric acid; HQC = hydroxyquinoline citrate; AAsc = ascorbic acid.
Experimental design
Distilled water was used for the preparation of the preservative solutions (treatments), which contained Sac + CA + HQC, AAsc or Crystal®, and the control was a group of tuberose flower spikes that were kept only in distilled water (Table 1). The pH and electrical conductivity (EC) of each solution was determined with the help of some sensors (LAQUAtwin pH-33 and CE-33, Horiba®, Japan). Inflorescences were placed under laboratory conditions (22 ± 2 °C, 60 % RH and 120 mmol·m2·s-1 light intensity). The experimental design was completely randomized, where source of variation was preservative solutions, the experimental unit was a tuberose flower spike, and there were eight replicates for measuring non-destructive variables and six replicates for destructive variables. Samples were taken every 2 d.
Response variables
One of the non-destructive variables was appearance, for which a hedonic scale was used where 5 was excellent (spikes with turgid flowers), 4 was good (spikes with edge of flowers with initial signs of dehydration), 3 was regular (spikes with first basal flowers withered), 2 was bad (spikes with 10 % of flowers withered) and 1 was considered senescent (flowers with more than 25 % of flowers withered); when flowers reached classification 2, they were considered without commercial value. Another variable evaluated was water consumption; for this purpose, inflorescences were placed in 1 L test tubes (with 500 mL of the preservative solution) without changing the water and the change in volume was recorded every 2 d until the end of the experiment. The relative fresh weight was measured in each tuberose flower spike using a digital scale (Ohaus®, USA), regarding 100 % the initial weight; this variable was determined in parallel with water consumption. The number of accumulated open flowers was obtained from each flower spike, from the basal part to the apical part.
Destructive variables were respiration or CO2 production, total sugars and specific superoxide dismutase (SOD) activity. Respiration was quantified by means of a static system (Salveit, 2016), which consisted in placing a tuberose flower spike in a plastic container with a capacity of 4 300 mL, hermetically sealed for 3 h. Subsequently, 1 mL of gas was taken from the headspace through a septum of the container and injected into a gas chromatograph (789A GC, Agilent Technologies, USA), which had an open-type column with porous silica layer gaskets, simultaneously connected to a thermal conductivity detector. Equipment conditions were 150, 80 and 170 °C for the injector, oven and detector, respectively. Nitrogen was used as carrier gas. CO2 quantification was performed with a standard of 460 mg·L-1 provided by Infra S.A. de C.V. (Mexico).
Total sugars were determined from the Antrone method (Whitam et al., 1971). A total of 1 g of two flower petals was taken from the middle of each flower spike and was finely chopped, 20 mL of 80 % alcohol was added and boiled for 10 min. The mixture was kept in refrigeration for 15 d; then, it was filtered, 1 mL of the solution was taken, evaporated and diluted (1:20); from which 1 mL was taken, the volume was adjusted to 3 mL with distilled water, 6 mL of Antrone reagent was added and the mixture was stirred (Vortex-Genie 2, Scientific Industries, USA). At the same time, a blank was prepared to which 3 mL of distilled water and 6 mL of Antrone reagent were added (0.4 g·L-1); the reaction was carried out in cold water. The tubes were brought to a boil in a water bath and then immersed in cold water. Finally, absorbance readings were taken at 600 nm in a spectrophotometer (Uv-vis GENESYS™ 10S, Thermo Fisher, USA). Sugars were quantified by means of a glucose calibration curve.
To determine the specific SOD activity, 10 flowers were taken from the middle to the apex of the flower spike, and the flower petals were used to make acetone powder (PAc) (Alia-Tejacal et al., 2005). PAc was obtained from 15 g of petals plus 25 mL of acetone in freezing (-15 °C), the mixture was homogenized (T 25 digital Ultra-turrax®, IKA, USA) for 25 s and vacuum filtered in a Buchner funnel. After homogenizing and filtering three times, the extract was left to dry in Petri dishes at room temperature (22 ± 2 °C); after 5 h, it was weighed and stored in the freezer (-20 °C) in plastic bags until further analysis. The weight of the PAc was determined according to the ratio of the fresh weight of macerated flowers to the weight of the dry powder.
From the PAc, the extraction and quantification of SOD activity was carried out (EC. 1.15.1.1). The procedure started from 0.1 g of acetone powder, to which 5 mL of Tris-HCl buffer (0.1 M, pH 7.8) were added and homogenized for 20 s; then, the mixture was placed in a centrifuge (Z 326 K, HERMLE, USA) at 12 096 g for 30 min at 4 °C and the supernatant was reserved. For the enzymatic test, the methodology proposed by Beyer and Fridovich (1987) was used, in which 27 mL of phosphate buffer (0.1 mM and pH 7.8 + EDTA), 1.5 mL of L-methionine (30 mg·mL-1), 1 mL of tetrazolium nitroblue (1.41 mg·mL-1) and 0.75 mL of 1% Triton X-100 were mixed. To 3 mL of this mixture, 0.5 mL of the supernatant and 0.03 mL of riboflavin (4.4 mg·100 mL-1) were added; the mixture was stirred and illuminated for 7 min with fluorescent light and, later, the readings were made in absorbance at 560 nm using a spectrophotometer.
The increase in absorbance, due to the formation of nitro-blue tetrazolium formazan (NBTF), per unit of time is equivalent to the reaction rate; absorbance in the absence or presence of various amounts of supernatant with SOD was used to determine the number of units per milliliter of SOD in solution. The enzymatic activity was reported as specific activity (U·mg prot-1), where U is an enzymatic unit of SOD and is equal to the amount of supernatant that photo-inhibits 50 % of the NBTF formation (Giannopolities & Ries, 1977; Stauffer, 1989).
Soluble protein was determined by the Bradford's method (1976). For this purpose, a mixture was prepared with 0.05 g of PAc and 5 mL of Tris-HCl (0.1 M, pH 7.8), and it was homogenized for 10 s. Then, the mixture was centrifuged at 12 096 g for 30 min at 4 °C. From the supernatant, 0.1 mL was taken and mixed with 5 mL of Coomassie blue solution. The mixture was stirred, and its absorbance measured at 595 nm after 12 min in a spectrophotometer. Quantification was done from a calibration curve with bovine albumin.
Data analysis
The data were subjected to an analysis of variance and a Tukey mean comparison (P ≤ 0.05). The SAS statistical package (SAS Institute, 1999) was used for data analysis, as well as the GLM and MEANS commands (Castillo-Márquez, 2011).
Results and discussion
The ‘Mexicano’ variety, with different preservative solutions, increased its relative fresh weight on the third day: 13 % with Crystal®, 9 % with Sac + CA + HQC and 5 % with AAsc. Afterwards, the relative fresh weight was kept in the case of tuberose flower spikes with Crystal®, and with the rest of the treatments it decreased more than 100 % (Figure 1 ). The ‘Perla’ variety had a significant increase between 11 and 18 % of fresh weight with Crystal® and AAsc, after 3 and 5 days of evaluation, respectively, being maintained even after 7 days (Figure 1B). Control flower spikes, both varieties ‘Mexicano’ and ‘Perla’, showed the lowest fresh weight from the third day (Figure 1A and 1B). In postharvest, the relative fresh weight behavior of cut flowers reflects the balance between the water they absorb and the water they lose through transpiration through leaf stomata and, in some cases, flowers (van Doorn, 2012). In both tuberose varieties, the solution with Crystal® favored water consumption and probably stoma closure.

Figure 1 Changes in relative fresh weight, water consumption, appearance and open flowers in two native varieties of tuberose: ‘Mexicano’ (A, C, E and G) and ‘Perla’ (B, D, F and H), submitted to different preservative solutions (Crystal®, sucrose [Sac] + citric acid [CA] + hydroxyquinoline citrate [HQC] or ascorbic acid). Each point represents the average of eight observations ± their standard error. The notations ns, *, ** and *** indicate statistical significance with 0.05, 0.01 and <0.0001, respectively. Numbers in black indicate minimal significant difference (Tukey, P ≤ 0.05).
For the variety ‘Mexicano’, water consumption was between 101 and 148 % higher for flower spikes treated with some nutritive solution compared to the control flowers (Figure 1C). In the case of the variety ‘Perla’, water consumption was between 101.5 and 238.9 % higher for flowers treated with Crystal® or AAsc, while flower spikes treated with Sac + CA + HQC consumed between 54 and 64 % more compared to the control flower spikes (Figure 1D). Solutions with Crystal® or AAsc improved water relations for both varieties (Figures 1A -D).
One of the main problems of the tuberose in post-harvest is the obstruction of the xylem (Sao & Verma, 2020). The preservative solutions evaluated favored water consumption because the pH of the water decreased (Table 1), which prevented the development of microorganisms in the environment and aided in water absorption (Kumar et al., 2017). Mosqueda-Lazcares et al. (2011) evaluated the use of Crystal® in eight rose varieties and observed an improvement in hydration, as there was a 69 % increase in relative fresh weight compared to stems placed in tap water. These authors attributed this response, in part, to the pH of the Crystal® solution being 5, which was lower than the pH of tap water, which was between 6.5 - 7, suggesting that the surface tension of the water was broken and absorption by the conductive tissues of the rose stem was easier. The preservative solutions evaluated showed higher EC (Table 1), suggesting that some of the solutes were transported to the leaves and petals, which caused higher water consumption (Sigma et al., 2018).
At the beginning, the appearance of the ‘Mexicano’ tuberose flower spikes was excellent, on the third day it was good and on the fifth day regular, with no differences between treatments (Figure 1E). In the case of the variety ‘Perla’, tuberose flower spikes treated with Crystal® showed an excellent appearance, even after 5 d. After 7 d of evaluation, the tuberose flower spikes with Crystal® or AAsc showed a regular appearance. On the other hand, the control flowers had a bad appearance from the fifth day. The application of Crystal® and AAsc generated a positive effect on the variety ‘Perla’ (Figure 1F).
The product Crystal® has been evaluated in roses and has improved their post-harvest life, mainly because it decreases pH and acidity, and provides sugars needed for flower opening (Mosqueda-Lazcares et al., 2011); a similar effect can be attributed to the tuberose. AAsc improves postharvest life in cut flowers because it is considered a bioregulator that in low concentrations has an effect on quality and vase life, mainly as a promoter of plant compounds (Budiarto, 2019).
The varieties ‘Mexicano’ and ‘Perla’ showed no significant differences between treatments in the number of flowers open at the beginning of the experiment (Figure 1G and 1H). Later, tuberose flower spikes of the ‘Mexicano’ variety placed in preservative solutions showed a greater number of flowers open at 5 d (between 24 and 28 flowers), compared to the control tuberose flower spikes (Figure 1 ). After 7 d, 13-14 ‘Perla’ flowers opened with Crystal® and AAsc (Figure 1H). In both varieties, the flower spikes treated with Crystal® or AAsc maintained the highest flower opening (Figure 1H). Anjum et al. (2001) mentioned that doses of AAsc between 50 and 200 mg·L-1 increased flower opening by up to 41 % compared to the control (14.21% opening), which is similar to that obtained in this study.
Roses have reported that AAsc helps to maintain flower opening, even after water stress (Jin et al., 2006). In some cut flowers, the application of sugars in solution favors the postharvest behavior and the floral opening (Reid, 2009). In the present study, the preservative solution composed of Sac + CA + HQC in the case of the variety ‘Mexicano’ also favored the floral opening (Figure 1G). This suggests that solutions are important in tuberose because of their effect on flower opening.
Respiration for the variety ‘Mexicano’ remained similar during the first three days of evaluation, and on the fifth day it increased in all treatments, except for the flower spikes with Crystal® (Figure 2A). For the variety ‘Perla’, respiration increased from the third day on the flower spikes with Sac + CA + HQC and the controls, while the flowers spikes with Crystal® reduced respiration until the fifth day of the evaluation, and on the last day it increased significantly. This situation did not occur in the flower spikes with AAsc, which had constant respiration (Figure 2B). Reid and Jiang (2012) indicate that post-harvest life of daffodil flowers is shorter when respiration increases, which occurred in the control flower spikes and with application of Sac + CA + HQC.
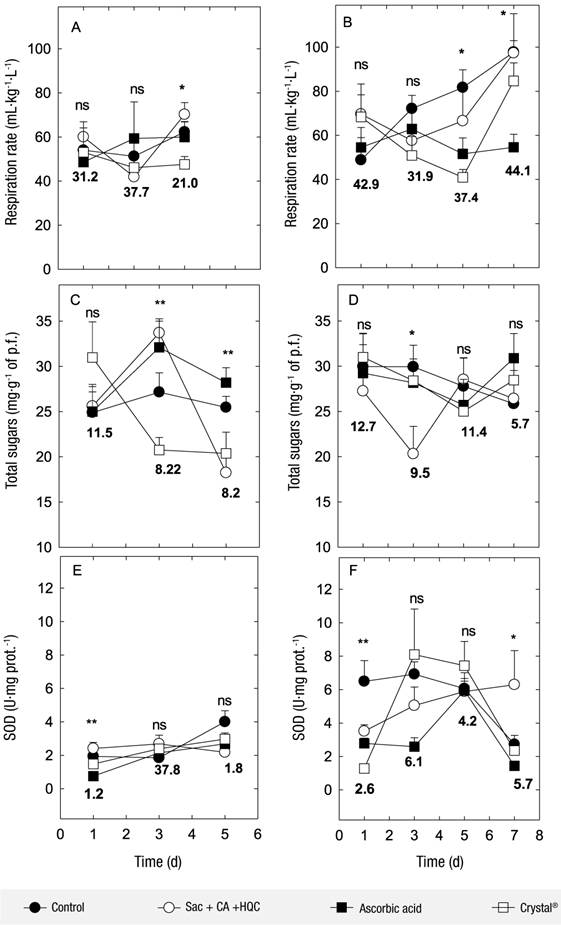
Figure 2 Changes in respiration, total sugars and specific superoxide dismutase (SOD) activity in two native varieties of tuberose: ‘Mexicano’ (A, C, E and G) and ‘Perla’ (B, D, F and H), submitted to different preservative solutions (Crystal®, sucrose [Sac] + citric acid [CA] + hydroxyquinoline citrate [HQC] or ascorbic acid). Each point represents the average of eight observations ± their standard error. The notations ns, *, ** and *** indicate statistical significance with 0.05, 0.01 and <0.0001, respectively. Numbers in black indicate minimal significant difference (Tukey, P ≤ 0.05).
In the concentration of total sugars, no significant differences were detected between the treatments applied to ‘Mexicano’ tuberose at the beginning of the experiment, but from the third day on, flower spikes treated with Sac + CA + HQC and AAsc had the highest concentration of sugars, and those treated with Crystal® maintained the lowest concentration (Figure 2C). On the fifth day, flower spikes with AAsc and the controls were maintained with the highest values, and flower spikes with Sac + CA + HQC and Crysal® with the lowest values (Figure 2C). The variety ‘Perla’ had differences only between treatments on the third day, where flower spikes treated with Sac + CA + HQC had the lowest sugar content. In the following days, no differences between treatments were detected, and the trend in all treatments was decreasing.
Arief-Zargar (2016) points out that during the senescence of the flowers the content of sugars in petals decreases because they are used as substrates for respiration. However, in the present study this association was not observed because in the case of the variety ‘Mexicano’ the flower spikes with lowest respiration also had the lowest concentration of sugars, which were those treated with Sac + CA + HQC. In contrast, flower spikes of the variety ‘Perla’ did not show differences in total sugar content, while in respiration clear differences were observed between treatments (Figure 2B). The lack of association between sugars and respiration is probably attributed to the fact that sugars were evaluated in flowers from the middle of the spike and respiration was evaluated from the whole spike.
For the variety ‘Mexicano’, the specific SOD activity at the beginning of the experiment was higher in the flower spikes treated with Sac + CA + HQC, and the lowest activity was in the flower spikes treated with AAsc. In the following days, no differences were detected between treatments, although it was observed that the flower spikes with preservative solutions increased their activity in a lower proportion compared to the control flower spikes, which was more evident on the fifth day of evaluation (Figure 2E). The variety ‘Perla’ showed differences between treatments in terms of specific SOD activity, where control flower spikes kept their activity constant for 5 d, and on the seventh day had a drastic decrease. On the other hand, the spikes with preservative solutions started with low activity and reached their maximum on the third, fifth and seventh day when they were treated with Crystal®, AAsc or Sac + CA + HQC, respectively. At the end of the period, this activity decreased dramatically in all treatments, except for the flower spikes treated with Sac + CA + HQC (Figure 2F)
Saeed et al. (2014) suggest that higher SOD activity in postharvest cut flowers is associated with a delay in senescence and therefore maintains adequate quality longer, resulting in longer post-harvest life. This behavior was mainly observed in ‘Perla’ flower spikes treated with Crystal® and AAsc.
Conclusions
Preservative solutions in tuberose varieties ‘Mexicano’ and ‘Perla’ favor water consumption, flower opening and SOD activity; in addition, they maintain fresh weight, good appearance for more time and decreases respiration. No effect of preservative solutions on sugar content was detected. Solutions with Crystal® or AAsc had a beneficial effect on the post-harvest behavior of ‘Mexicano’ and ‘Perla’ tuberose flower spikes, which can be considered for developing post-harvest management technologies.











 texto en
texto en 


