Introduction
The demand for food products free of artificial colors is growing because consumers perceive them as healthier (Martins, Lobo-Roriz, Morales, Barros, & Ferreira, 2016). Anthocyanins are pigments of vegetable origin that exhibit a range of hues from pink to deep purple, and have high water solubility, which facilitates application in food. However, their use as colorants is limited by low stability to factors such as: pH, temperature, light and oxygen; elements present during food processing and transformation (Turturică, Oancea, Râpeanu, & Bahrim, 2015). The microencapsulation technique reduces instability of these pigments (Özkan & Biley, 2014), so studies have multiplied on incorporation of microencapsulated anthocyanins in food products as a way to enhance color (Díaz-García et al, 2014), provide new hues (Šaponjac et al., 2016; Vernon-Carter et al., 2020) and improve functional value (Mahdavi, Jafari, Assadpoor, & Ghorbani, 2016).
Mexico, as a center of origin and domestication of maize, has a wide diversity in this cereal, which is manifested in different colors, textures and grain sizes; blue-purple maize grains are part of this diversity. Its color is due to the presence of anthocyanins in aleurone (Salinas-Moreno, Pérez-Alonso, Vázquez-Carrillo, Aragón-Cuevas, & Velázquez-Cardelas, 2012), and they are used for the elaboration of diverse food products with natural coloring.
Blue native maize tortillas are appreciated by the consumer due to pleasant taste, color and texture, which positions them as a special product marketed at a higher price than the white grain maize tortillas (Chimimba, Pratt, Cuellar, & Delgado, 2019). However, most of the native blue-purple maize grains have floury texture, so dough lacks the necessary resistance to make tortillas in a mechanized manner. Blue-purple maize grains, with intermediate or hard grain texture, produces dough with the texture required for mechanized processing, but has lower anthocyanin content than floury maize grains, and tortillas have a lower amount of antioxidants and a blue-gray color (Hernández-Martínez et al., 2016).
To increase the supply of blue tortillas in the market, nixtamalized white maize flour can be supplemented with microencapsulated anthocyanins, which generates tortillas dyed with natural pigment (Vernon-Carter et al., 2020), but with a different color than the ones consumers are used to from blue tortillas. Another option is to add anthocyanins to the native maize dough with dentate blue-purple grains to increase antioxidant content of tortillas, and thus achieve a color similar to that of the traditional blue tortilla.
Therefore, the objective of this study was to evaluate the addition of anthocyanins in microcapsules (MC) or extract (EL), to the dent blue-purple maize dough on color, texture, phenolic composition and antioxidant capacity (AC) of the maize tortilla. The hypothesis of this study was that addition of anthocyanins to blue-purple maize dough allows obtaining tortillas with a blue color similar to the blue tortillas from central Mexico.
Materials and methods
Plant material
The study used native maize populations with purple and blue-purple grains. The purple grain population was collected in San Salvador Ixtenco, Tlaxcala, Mexico, and was used for extraction of pigments. Purple maize grains have, on average, 50 % more pigment than blue-purple maize grains. To obtain the dough in which anthocyanins were added, the blue-purple grain of variety VC-42 grown in Etla, Oaxaca, Mexico was used. This variety is derived from the Bolita (Bol) variety of maize and has a semi hard grain that produces dough with the necessary texture for mechanized processing. However, its low anthocyanin content gives the dough and the tortilla a grayish blue color (Hernández-Martínez et al., 2016), different from the characteristic color of the blue tortilla obtained from native Cónicos or Chalqueños maize grains, which are the most used to make blue tortillas in the central region of Mexico. As a reference for the blue tortilla color, blue-purple maize from the Chalqueño (Chal) variety from San Pedro Nexapa, Estado de Mexico, was used. This tortilla was also used as a reference for antioxidant content.
Pigment extraction from purple maize grain
After removing the germ manually, 1 kg of maize grain was ground using a cyclone mill (UDY Corporation, USA) with 0.5 mm mesh. The extraction batches consisted of 100 g of flour and 500 mL of 63.5 % ethanol as solvent, the latter acidified with citric acid at a pH of 2 ± 0.2. The subsequent extraction steps were performed as described by Salinas-Moreno, Salas-Sánchez, Rubio-Hernández, and Ramos-Lobato (2005). The anthocyanin extract was concentrated in a rotary evaporator (R-215, Buchi, Sweden) until ethanol was removed. This aqueous extract (with pH of 2) was used to elaborate MC and for treatments of addition of anthocyanins in EL form.
Microencapsulation of anthocyanins
EL of anthocyanin was adjusted to a pH of 4 ± 0.2 with NaOH using a potentiometer (ɸ 45 pH Meter, Beckman). The methodology described by García-Tejeda, Salinas-Moreno, Hernández-Martínez, and Martínez-Bustos (2016) was followed to obtain MC by spray drying. As wall material, 20 g of a 50:50 Capsul®:maltodextrin (DE = 10) mixture were used, which were dispersed in water and extract. Preparation was homogenized in an Ultra turrax (T-25-SI, Ultra Turrax®, USA) at 18 000 rpm for 5 min. Emulsion was adjusted to 25 % total solids with a refractometer (Atago®, Japan). Encapsulation was performed in a spray dryer (Mini Spray Dryer B-191, Buchi) with air inlet and outlet temperatures of 180 ± 1 °C and 100 ± 5 °C, respectively, pressure of 35 psi, nozzle diameter of 0.5 mm and feed flow of 10 mL·min-1. Powders were collected in glass bottles protected from light with aluminum foil and stored in a desiccator at room temperature.
Total and surface anthocyanins of microcapsules
Total anthocyanin content (TAC) in MC was performed as described by Salinas-Moreno et al. (2005), with adjustments in the sample size (20 mg) and sonication time (20 min) to ensure the complete breakdown of MC and release of anthocyanins. Surface anthocyanins were determined using Robert et al. (2010) methodology, in which MC are washed with ethanol and aqueous methanol, filtered with Whatman No. 4 and the filtrate is analyzed to obtain TAC.
Nixtamalization of blue-purple maize grain samples
Nixtamalization of blue-purple maize grains was carried out in 1 kg grain lots according to the methodology described by Salinas-Moreno and Vazquez (2006). The cooking time was assigned according to the grain hardness measured by the flotation index (25 min for maize Chal and 45 min for maize Bol). Resting time was 12 h at room temperature. The nixtamal was washed with running water and passed through a stone mill. The dough of maize Chal was conditioned with purified water to elaborate the tortillas from 20 g of dough. A portion of this maize dough was used as a color reference for pigment addition treatments. Treatments were adjusted to the amount of pigment added, so that the color of the dough was equal to, or as close as possible to, that of the Chal maize dough.
The dough was molded with discs of 11 cm in diameter using a manual metal press. The cooking was done in a metallic comal heated with butane gas at an average temperature of 240 ± 5 °C. The dough of maize Bol was separated in 200 g portions, which constituted the experimental unit on which the treatments of addition of pigments were applied. The dough of this maize without added pigments and tortillas were used as control of the treatments added with anthocyanins.
Anthocyanin addition treatments
The addition of anthocyanins was done in the form of MC or EL on 100 g samples of Bol maize dough. Surface anthocyanins in the form of MC were 7.36 ± 0.29 µg cyanidine-3-glucoside equivalent (ECG) per gram of MC, while total anthocyanins amounted to 1 090.7 ± 142.8 µg ECG·g-1 of MC. Previous analysis was carried out with different amounts of MC in dough and its color was visually compared with that of the reference dough (Chal). These tests allowed defining the range of MC quantities to be used, which was established in 0.5, 0.75 and 1.0 % in weight (corresponding to T1, T2 and T3, respectively). Thus, 0.5 g of MC were added to 100 g of dough, and so on. According to the content of anthocyanins in MC, 0.5 g of MC contained 0.5453 mg of anthocyanins, 0.75 g was equivalent to 1.0907 mg and 1 g corresponded to 1.636 mg. These same amounts of anthocyanins were added in the form of EL, so that treatments were: 0.5 % EL (T4), 0.75 % EL (T5) and 1.0% EL (T6). All treatments were carried out in duplicate.
Dough moisture content varied from 50 to 52 %. Once anthocyanins (MC or EL) were added, the dough was manually homogenized to integrate anthocyanins, adding purified water to reduce hardness, and it was left to rest in plastic bags for 20 minutes before making tortillas under the procedure described above. A portion of dough from each treatment was separated to determine color, moisture, TAC and total soluble phenols (TSP). The tortillas were placed on a piece of cotton blanket and let cool at room temperature before being refrigerated.
Color and moisture in dough and tortilla
The color was measured using a Minolta colorimeter (CM-5, Konica Minolta) in CIELab scale. The parameters obtained were luminosity (L*, black/white), a* (red/green), and b* (yellow/blue). A 20 g portion of dough was placed on a glass surface to obtain readings. In the case of the tortilla, it was exposed directly on the reader of the equipment with the opposite side where the tortilla expands. The color of the dough and the tortilla were measured on the day of preparation. For the tortilla, this determination was repeated after 7 days of refrigerated storage. Hue angle [H* = arctag(b*/a*)] and chroma [C* = √(a*2 + b*2)] were calculated as described by Jha (2010). Moisture content in dough and tortilla was determined by the method 44-10 of the American Association of Cereal Chemists (AACC, 2000).
Tortilla texture
Texture was determined in the tortilla stored for 24 h under refrigeration. This is due to the fact that retrogradation of starch in tortilla accelerates at low temperatures (Bueso, Waniska, Moreira, Seetharaman, & Rooney, 2006), and greater changes in texture occur within the first 24 h of elaboration (Campas-Baypoli, Rosas-Burgos, Torres-Chávez, Ramírez-Wong, & Serna-Saldivar, 2002). To determine texture, the tortilla was heated in a microwave oven for 30 s at medium intensity, in blocks of three tortillas wrapped in a cotton blanket and inside a plastic bag, which was placed in a plastic container to maintain the temperature of the tortilla between 35 and 40 °C. From the central part of the tortilla, a 4 x 9 cm rectangle was cut and placed between the ends of the “tensile grips” tensor of the texture analyzer (Texture Analyser TA-XT2, Stable Micro Systems, England). The test speed was 1 mm·s-1, with a distance of 40 mm (Suhendro, Almeida-Domínguez, Rooney, Waniska, & Moreira, 1999). The parameters determined were force at 1 mm extensibility (FE; N), shear force at tension (FC; N) and extensibility distance (E; mm). For each treatment, four to five measurements were taken.
Total soluble phenols and anthocyanins
Portions of dough and tortilla, from the different treatments, were placed in aluminum trays and dehydrated for 15 h at 37 °C in a drying oven, and then ground using a cyclone-type mill with 0.5 mm mesh. Phenolic compounds were extracted as described by Salinas-Moreno et al. (2005) from 1 g of ground sample and 20 mL of methanol (J.T. Baker®, Mexico) acidified to 1 % with triflouracetic acid (Sigma Aldrich, USA). The mixture was sonicated for 15 min in a sonicator bath (model 2510, Branson, USA) and refrigerated to complete 2 h of extraction. Subsequently, it was centrifuged (Universal 32, Hettich, Germany) at 2 660 g for 10 min, and the supernatant was filtered using Whatman paper No. 4 and its volume was measured. TSP, TAC and AC were determined from this extract.
The quantification of TSP was carried out by the method of Folin-Cicalteau (Singleton & Rossi, 1965). A standard curve of ferulic acid (Sigma-Aldrich, USA) was prepared and the results were expressed in milligrams of ferulic acid equivalent per gram of dry sample (mg EAF·g-1 MS). TAC was obtained according to Salinas-Moreno et al. (2005); for this purpose, a cyanidine-3-glucoside (Extrasynthese, France) standard curve was prepared. The results were expressed in milligrams of cyanidine 3-glucoside equivalent per gram of dry sample (mg ECG·g-1 MS). The analyses of these variables were carried out in duplicate.
Antioxidant capacity
The AC of the tortillas was determined by two methods to achieve a more complete assessment, given the diversity of compounds present in the extract. The ABTS method (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) was carried out according to Re et al. (1999), and the FRAP method (iron reducing antioxidant power) was carried out according to Benzie and Strain (1996). In both methods, standard Trolox curves were run to express the results in µmol of this compound equivalent per gram of dry matter (µmol ET·g-1 MS).
Statistical analysis
Data was analyzed under a completely randomized experimental design, and the results obtained were subjected to an analysis of variance to evaluate the effect of treatments and Tukey mean comparison test (P ≤ 0.05). The SAS statistical package version 9.0 (SAS Institute, 2002) was used for this purpose.
Results and discussion
Color in dough and tortilla
The reference dough (Chal) had a dark blue-green color, with L* values of 50.2 ± 0.2 %, H* of 204.1 ± 0.9° (Figure 1a) and C* of 5.4 ± 0.1 (Figure 1b). On the other hand, the control dough (Bol) was less dark (L* = 56.9 ± 0.1 %), greenish hue (H* = 190.4 ± 1.3°; Figure 1a) and less blue (C* = 3.7 ± 0.02) compared to the reference dough (Figure 1b). The addition of anthocyanins to the dough in MC at medium (0.75 %) and high (1.0 %) concentrations changed the color to H* values of 295.7 ± 3.1 at 302.2 ± 1.2°, and C* of 0.66 ± 0.03 at 1.1 ± 0.1, respectively, without changing L* (Figure 1a), so the dough hue became blue-purple (Figure 1b). With the lowest MC level, a colored dough closer to the reference dough was achieved (DE = 7.19, data not shown).
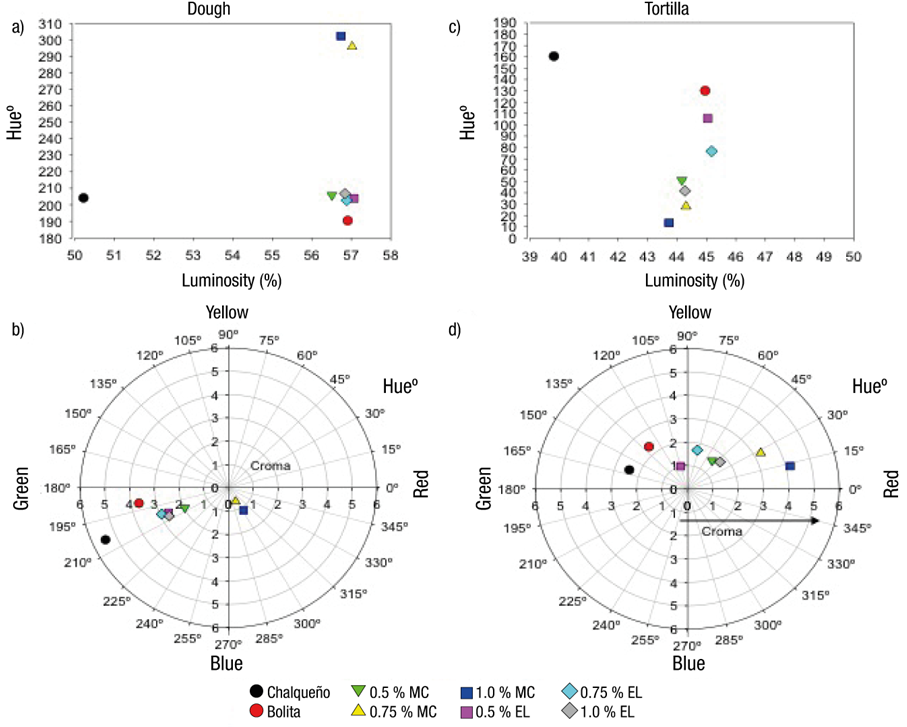
Figure 1 Distribution in the hue-luminosity (a, c) and hue-chromaticity (b, d) diagrams of dough and tortilla color data of treatments with addition of pigments and the reference sample (Chal). MC = microcapsules; EL = extract; Bolita = control without pigments.
When the pigment was added in EL, changes in the color variables (L*, H* and C*) of the dough, with the three levels of anthocyanins, were minimal (Figure 1b). This result is attributed to the fact that, when adding anthocyanins in EL, the contact of flavonoids with alkaline matrix that represents the dough, is immediate, which causes the chemical form of flavilium cation (intense red color predominant at acid pH) to change to that of ionized chalcone (straw yellow color), which is unstable (Brouillard, 1982). By adding anthocyanins in MC, the wall material surrounding them protects them from alkaline pH.
The color of the dough with anthocyanins added in MC or EL changed with the heating applied to obtain the tortilla. The luminosity was reduced as a result of the loss of water through cooking (Figure 1c), and hue values were located in the first and second quadrants of the color diagram (Figure 1d). The tortillas of the reference sample (Chal) and control (Bol) were located in the second quadrant, with Hue values of 160.5 and 130.1º, respectively, corresponding to green-yellow hues. The chroma was 2.4 for both tortillas, so the color was not very bright. The color of the dough was not a good predictor of the color of the tortilla in the treatments added with anthocyanins. This is because, although color was similar to the reference dough (with values of ΔE between 7 and 8; data not shown), the tortillas have a different color, which was characterized by reddish hues, particularly those added with anthocyanins in MC (Figure 1d). Tortillas with anthocyanins in EL showed red-orange to yellow-green hues, with H* values from 41.7 to 105.6° and C* from 1.0 to 1.7, so appearance was opaque (Figure 2).
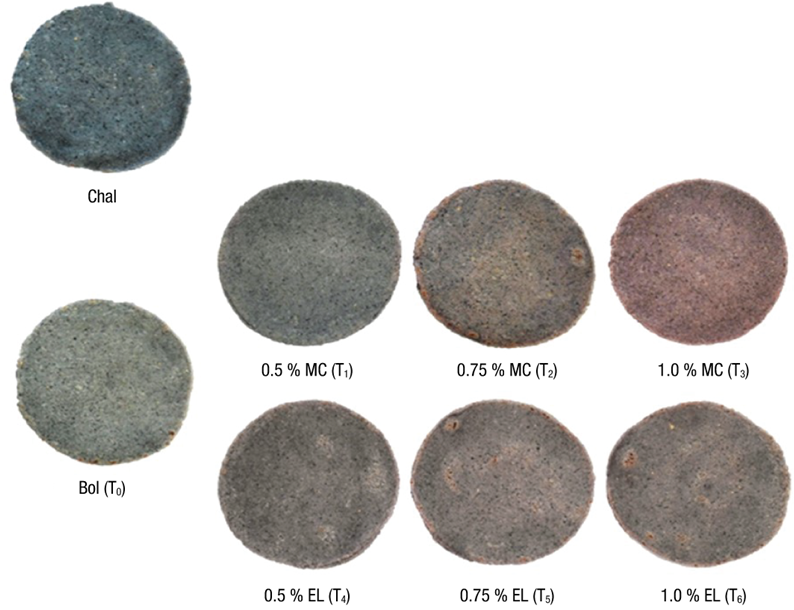
Figure 2 Images of tortillas with anthocyanin treatment in the form of microcapsules (MC) or extract (EL). Bol = Bolita maize tortilla without addition of anthocyanins; Chal = tortilla made with Chalqueño maize used as color reference.
Tortillas with anthocyanins in MC stored for 7 days under refrigeration conditions showed higher L* values compared to freshly made tortillas (day 0). In contrast, anthocyanins in EL reduced L* values, so they were less luminous and darker compared to freshly made tortillas (Figure 3a). Cookies with MC of bitter cherry (Prunus cerasus) anthocyanins had an increase in L* values during storage (Šaponjac et al., 2016). On the other hand, Jimenez-Lopez et al. (2019) observed no change in L* during storage for 7 days of waffles added with EL of anthocyanins obtained from strawberry tree (Arbutus unedo). In the first case, the results of L* in tortillas added with anthocyanins in MC coincide with that reported in cookies (Šaponjac et al., 2016); but in the second case they do not coincide, which can be attributed to the pH of the matrix in which anthocyanins were found in one case and in the other. Tortillas are an alkaline pH product (from 8 to 8.8) (Vázquez-Carrillo, García-Lara, Salinas-Moreno, Bergvinson, & Palacios-Rojas, 2011), while waffles have slightly acidic pH (from 5.5 to 6), so it is possible that the degradation of anthocyanins during storage has been more accelerated in tortillas because they are unstable at alkaline pH.
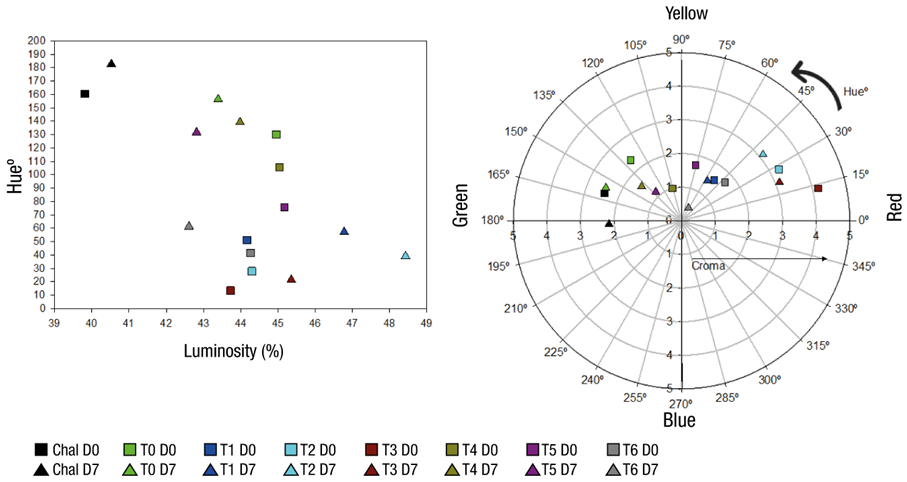
Figure 3 Distribution in hue-luminosity (a) and hue-chromaticity (b) diagrams of the tortilla color data, stored for 7 days, of the treatments added with anthocyanins in the form of microcapsules (MC) or extract (EL). Chal = tortilla made with Chalqueño maize used as color reference.
H* values increased with the storage of tortillas (Figure 3a). This increase was associated with the level of anthocyanins added to the dough. That is, the more anthocyanins in MC the tortilla has, the more the color changed from reddish-purple (day 0) to red-orange (day 7) (Figure 3b). The increase in H* values could be due to degradation of anthocyanins during storage, which was attributed to alkaline pH of the product. C* values were slightly reduced with the storage of tortillas, so their grayish appearance was more accentuated compared to freshly made tortilla.
Tortilla texture
Texture profiles of tortillas, including reference sample (Chal) and control Bol (T0), are shown in Figure 4. A marked difference was observed between the texture profile of the reference tortillas (floury grain) and those of the control (dent grain). Chal maize tortillas were characterized by low values of strength at 1 mm (FE) and FC, which were lower (P ≤ 0.05) than those of the control tortillas. Extensibility of Chal and Bol tortillas was statistically equal (P ≤ 0.05) (Table 1). Texture differences between Chal and Bol tortillas are attributed to the hardness of the grain of each maize, which is related to protein content and predominant type of starch (Santiago-Ramos, Figueroa-Cárdenas, Véles-Medina, & Mariscal-Moreno, 2017). Chal maize has a soft grain and lower protein and amylose content than Bol maize, which has a hard grain (Salinas-Moreno et al., 2012). Protein-starch interactions in hard maize grains are stronger than in soft maize grains, making soft maize tortillas less resistant to breakage compared to hard maize grains (Osorio-Díaz et al., 2011). However, Salinas-Moreno and Aguilar-Modesto (2010) found no statistical difference in FC between tortillas with soft maize grains (floury) and hard maize grains.
Table 1 Mean of texture variables in tortillas with Bolita maize (Bol) added with anthocyanins in the form of microcapsules (MC) or extract (EL).
| Treatments | Extensibility strength (N·mm-1) | Cutting strength (N) | Extensibility (mm) | Tortilla moisture (%) |
|---|---|---|---|---|
| Reference (Chal) | 0.57 bz | 2.19 d | 7.0 ab | 43.4 ed |
| Control (T0) | 1.11 ab | 3.51 ab | 10.4 ab | 46.9 abc |
| 0.5 % MC (T1) | 1.39 ab | 3.41 bc | 9.0 ab | 48.5 ab |
| 0.75 % MC (T2) | 1.49 ab | 2.45 cd | 5.0 b | 44.6 cde |
| 1.0 % MC (T3) | 1.67 ab | 3.19 bcd | 5.8 ab | 49.2 a |
| 0.5 % EL (T4) | 1.10 ab | 3.69 ab | 11.0 ab | 41.8 e |
| 0.75 % EL (T5) | 1.53 ab | 4.08 ab | 10.0 ab | 46.1 bcd |
| 1.0 % EL (T6) | 1.87 a | 4.46 a | 12.5 a | 47.5 ab |
| LSD | 1.3 | 1.04 | 7.3 | 2.8 |
Chal is the tortilla with Chalqueño maize used as reference, and T1 a T6 are the different treatments with addition of pigments; LSD = least significant difference. zMeans with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
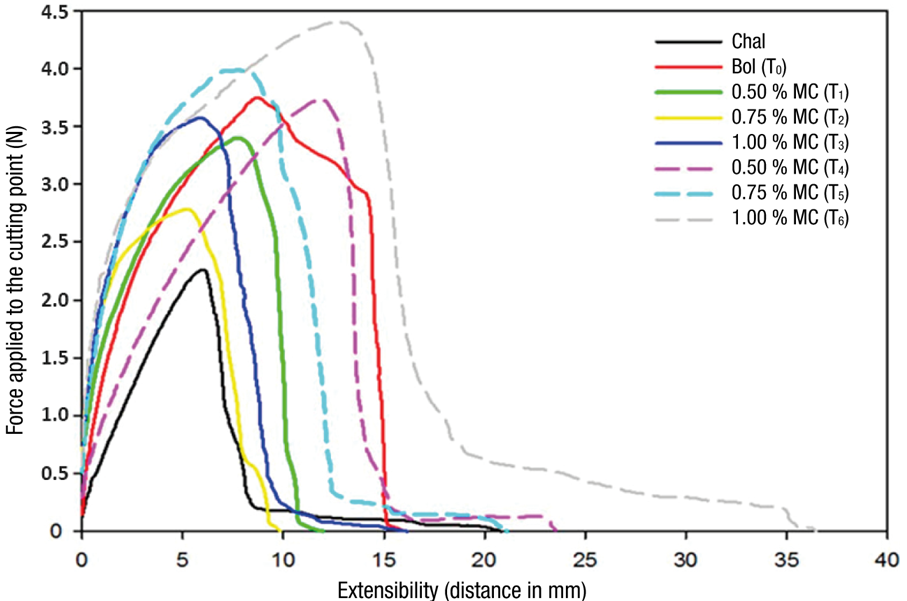
Figure 4 Texture of tortillas with Bolita maize (Bol) added with anthocyanins in the form of microcapsules (MC) or extract (EL). Chal is the tortilla made with Chalqueño maize used as reference, and T1 to T6 are the different treatments with addition of pigments.
The addition of anthocyanins to dough did not affect FE of the tortillas, because there was no statistical difference between control tortillas and treatments. However, a trend towards higher FE was observed by increasing the amount of anthocyanins added in both MC and EL. FC ranged from 2.19 to 4.46 N, where the tortillas with the highest values were those with anthocyanin added by EL, and the lower values corresponded to tortillas with anthocyanins in the form of MC (Table 1). Of the texture variables, FC is related to the hardness of the tortilla, and can be measured by tension, as in this study, or by puncture, as Vernon-Carter et al. did (2020). These authors, when adding MC of anthocyanins of purple maize to nixtamalized white maize flour in concentrations of 30, 60 and 120 mg·100 g-1 of dough to obtain naturally dyed tortillas, observed a hardness reduction when increasing the amount of MC added to the flour, and attributed this result to the interaction between tortilla's starch and polyphenols from MC that contained 25 % of anthocyanins.
The addition of anthocyanins to the dough, in MC or EL, did not alter the extensibility of tortillas, which was statistically the same (P ≤ 0.05) between treatments. However, there was a trend of lower extensibility in MC treatments, which could be due to the effect of polysaccharides in the wall material.
Tortilla moisture is related to its hardness, the higher the moisture the lower the hardness (Salinas-Moreno & Aguilar-Modesto, 2010). Tortilla moisture values varied from 41.8 to 49.2 %, with the lowest value in tortillas of T4 and the highest values in tortillas from treatments T3 and T6. However, no clear relationship was observed between anthocyanin levels and the way they were added (MC or EL) with tortilla moisture.
FC values of blue-purple maize tortillas with and without added pigments are within those reported by Cruz-Chávez, Cadena-Iñiguez, Salinas-Moreno, and Garrido-Ramírez (2013), which were 1.96 to 5.88 N for tortillas obtained from different accessions of Mexican maize, with the same grain color and evaluated under tortilla temperature conditions similar to the present study. When FC evaluation is performed on cold tortilla, values are considerably elevated due to tortilla stiffness. This was demonstrated by Chávez-Santoscoy, Gutiérrez-Uribe, Serna-Saldivar, and Pérez-Carrillo (2016), who reported FC values of 10.2 to 10.4 N in maize tortillas with the addition of black bean seed coat extract.
Total anthocyanins, total soluble phenols and antioxidant capacity in dough and tortilla
Phenolic composition variables (TAC and TSP) and AC of dough and tortilla are shown in Table 2. The incorporation of anthocyanins to dough in the form of MC or EL increased TAC to values of 26.1 mg ECG·100 g-1 MS, but without equaling the value observed in the reference dough (32.4 mg ECG·100 g-1 MS). When the pigment was added in the form of MC, TAC increased as the concentration increased. However, when it was added as EL, the increase was observed only with the highest concentration, which is attributed to the possible degradation of anthocyanins by alkaline pH of dough, which was not observed in the low and medium concentrations due to the low amount of added anthocyanins. TSP showed a similar behavior to that observed in TAC.
Table 2 Mean values of total anthocyanins (TAC), total soluble phenols (TSP) and antioxidant capacity (ABTS and FRAP) in blue-purple maize tortilla and dough added with anthocyanins in microcapsules (MC) or extract (EL).
| Treatment | TAC | TSP | ABTS | FRAP | Loss of anthocyanins (%) |
|---|---|---|---|---|---|
| Dough | |||||
| Reference (Chal) | 32.4 az | 148.0 a | 14.1 a | 7.2 a | |
| Control (T0) | 21.0 e | 124.9 c | 12.3 b | 5.5 c | |
| 0.5 % MC (T1) | 24.1 cd | 122.1 c | 13.1 ab | 5.9 b | |
| 0.75 % MC (T2) | 24.6 bc | 127.2 bc | 13.9 ab | 6.2 b | |
| 1.0 % MC (T3) | 25.8 b | 132.8 b | 13.1 ab | 6.2 b | |
| 0.5 % EL (T4) | 22.9 d | 106.7 d | 10.5 c | 5.4 c | |
| 0.75 % EL (T5) | 22.9 d | 122.2 c | 12.6 ab | 6.1 b | |
| 1.0 % EL (T6) | 26.1 b | 122.3 c | 13.3 ab | 5.9 b | |
| LSD | 1.7 | 6.2 | 1.6 | 0.4 | |
| Tortilla | |||||
| Reference (Chal) | 16.1 a | 78.9 bc | 7.9 d | 4.2 b | 50.2 |
| Control (T0) | 12.2 f | 69.9 d | 7.6 e | 3.7 f | 41.3 |
| 0.5 % MC (T1) | 12.9 e | 70.1 d | 8.0 d | 4.1 c | 46.5 |
| 0.75 % MC (T2) | 15.0 c | 79.7 b | 8.2 c | 4.0 d | 39 |
| 1.0 % MC (T3) | 15.5 b | 81.9 a | 8.9 b | 4.2 b | 39.9 |
| 0.5 % EL (T4) | 11.6 g | 59.4 e | 7.5 e | 3.3 g | 49.1 |
| 0.75 % EL (T5) | 14.0 d | 77.3 c | 8.3 c | 3.8 e | 38.8 |
| 1.0 % EL (T6) | 15.5 b | 82.5 a | 9.8 a | 4.4 a | 40.5 |
| LSD | 0.5 | 2.1 | 0.3 | 0.1 | |
TAC = milligrams of cyanidine 3-glucoside equivalent per 100 g of dry matter (ECG·100 g-1 MS); TSP = milligrams of ferulic acid equivalent per 100 g of dry matter (mg EAF·100 g-1 MS). ABTS and FRAP values are expressed in µmol Trolox equivalent per gram of dry matter (µmol ET·g-1 MS); LSD = least significant difference. Chal = Chalqueño maize used as reference. zMean with the same letter within each column do not differ statistically (Tukey, P ≤ 0.05).
When assessing AC from the dough, the ABTS method reported higher values compared to the FRAP test, which is attributed to a greater affinity of the phenolic compounds in the extract for the radical of the ABTS method (Žilić, Serpen, Akıllıoğlu, Gökmen, & Vančetović, 2012). It is also possible that the mechanism by which anti-radical activity is analyzed in both techniques has some involvement. The ABTS method shows both electron transfer and proton exchange (transfer of a hydrogen atom), while the FRAP method shows only electron transfer as it is an oxide-reduction method (Karadag, Ozcelik, & Saner, 2009).
With the ABTS and FRAP tests, the highest AC from the dough was in the reference (Chal), and the lowest in treatment T4. The addition of anthocyanins in MC or EL form to the dough of maize Bol increased AC values, but it was not enough to equal the value of the dough used as reference. This result is attributed to the fact that the amounts of anthocyanins added were very low (0.5453 to 1.636 mg·100 g-1 of dough), because efforts were made to keep the color of the tortilla as close as possible to the reference color.
When the dough was transformed into tortillas, TAC (38.8 to 50.2 %) and TSP (32.5 to 46.7 %) values were reduced due to high temperature (200 to 240 °C) during this process. Although the exposure time to high temperature was less than 1 min, the thickness of the tortilla (1.5 to 2 mm) causes a high percentage of phenolic compounds to degrade. TAC values were between 12.3 and 16.1 mg ECG·100 g-1 MS, while TSP varied from 59.4 (T4) to 82.5 mg EAF·100 g-1 MS (T6). The values obtained in phenolic variables (TAC and TSP) are similar to those reported by Hernández-Martínez et al. (2016) in tortillas from populations of blue-purple maize grain of Chalqueño and Bolita varieties.
TAC and TSP values in the tortilla increased as the amount of anthocyanins added to the dough increased. The highest values in both variables were found in tortillas with the highest concentration added, both in MC and EL. The loss of anthocyanins when transformed into tortillas was 41.4 and 50.2 %, respectively for reference dough and control treatment. While losses varied from 39.1 to 49.1 % for dough added with pigments, without any relation between the mode the pigment was added (MC or EL) and stability of anthocyanins at high temperatures. Azzurra-Papillo et al. (2018) used black rice anthocyanins in EL or MC in the formulation of biscuits to add color and enrich the product with bioactive compounds. Baking caused between 33 and 49 % of anthocyanin losses, with moderate protection of anthocyanins at baking temperatures when added in MC. Stability of anthocyanins at elevated temperatures is influenced by the pH of the matrix in which they are found. According to Li et al. (2013), anthocyanins are more stable at acidic pH.
The losses of anthocyanins when changing from dough to tortillas were higher than those reported by Chávez-Santoscoy et al. (2016) for maize tortillas added with black bean coats bioactives (12 to 20 %), and to those obtained by López-Martínez, Parkin, and García (2011) for blue-purple maize grains (32 %). The differences can be attributed to the cooking time of the tortilla, because when produced in a traditional way this variable is difficult to standardize. However, de la Parra, Serna-Saldivar, and Liu (2007) report a 44 % increase in the content of these compounds when changing from dough to tortilla, and Mendoza-Díaz et al. (2012) report a 111.9 % increase. Dough has a higher moisture content than tortilla, so the anthocyanin content for both products should be reported on a dry basis to make comparisons. De la Parra et al. (2007) indicate that the values are on a dry basis, while Mendoza-Díaz et al. (2012) do not mention it. However, an increase in anthocyanin content of tortillas in relation to that of dough is difficult to explain, due to susceptibility of anthocyanins to temperature (Patras, Brunton, O’Donnell, & Tiwari, 2010).
Anthocyanins represent a significant proportion of TSP, so changes observed in the latter variable were in the same direction as those of TAC, although magnitudes varied slightly. In the reference sample (Chal) and control (Bol), reductions were 46.7 and 44.0 %, respectively; while in treatments with addition of pigments the values varied from 32.5 to 44.3 % (in T6 and T4, respectively).
The addition of pigments to the dough improved the AC of the tortillas. According to the results of the ABTS method, all treatments equaled or exceeded the AC of the reference and control tortillas, except for T4, which was the same as the control. With the FRAP test, only treatments T3 and T6, which correspond to the highest concentration of anthocyanins added in MC and EL, respectively, equaled or exceeded the CA of the reference tortillas. These results differ from those obtained in dough, where none of the treatments added with anthocyanins equaled the CA of the Chal maize dough used as reference. This could be related to the loss of anthocyanins during tortilla cooking, because Chal maize dough lost the most anthocyanins.
The ABTS method was more sensitive compared to the FRAP method to changes in the anthocyanin content of the tortilla, as AC values with ABTS were reduced similarly to TAC. However, with the FRAP method, AC of the tortilla was reduced by an average of 34 % compared to the dough.
In experiments on the enrichment of food products with bioactives to increase AC, the amounts added do not consider the final color of the product (Chavez-Santoscoy et al., 2016; Azzurra-Papillo et al., 2018). However, when a color reference is established, as was the case with the Chal maize tortilla, the addition of bioactives is restricted, so the gain in AC with respect to the control was moderate (17 to 29 %).
Conclusions
The addition of anthocyanins in the form of microcapsules or extract to the blue-purple maize dough modified the color of the tortilla and increased the contents of anthocyanins and total soluble phenols, and antioxidant capacity. Tortilla hardness increased with the addition of anthocyanins in extract, although other texture variables were not affected. With high concentrations of anthocyanins, both in microcapsules and in extract, the color of the tortillas was different from that of the reference sample, presenting reddish tones.











 texto em
texto em 


