Introduction
Edible oils have been used throughout the world since ancient times and their production sources are very diverse, which generates a high variation in the characteristics of each oil type. An important source of oil is grape (Vitis vinifera L.) seed, as the world production of this fruit is estimated at 66 million tons per year. Generally, the fruit of the grape is consumed fresh or used to make jams, jellies, raisins, vinegar, and about 70 to 80 % of the total harvest is used in winemaking, where it is obtained as a by-product of the seeds of the fruit (de Haro, Izarra, Rodríguez, Pérez, & Carmona, 2016; Rombaut et al., 2015). These seeds represent 20 to 25 % of the biomass generated by the wine industry, so more than 3 million tons of grape seeds are discarded worldwide every year, making them an important agro-industrial waste (Coelho, Filipe, Robalo, ( Stateva, 2018; Fernández, Casal, Cruz, Pereira, & Ramalhosa, 2013).
Due to the health benefits of grape seed oil (GSO), such as a protector against cardiovascular diseases and as an anti-inflammatory, antioxidant, and blood cholesterol reducing agent, among others, its production has been proposed as an alternative to take advantage of the residues of wine companies. The oil content in grape seed is 10 to 20 %, with high concentrations of unsaturated fatty acids such as linoleic (58 to 78 %), oleic (3 to 15 %) and linolenic (0.3 %) (Kim et al., 2010), as well as tocopherols and tocotrienols (from 499 to 1 575 mg·kg-1 of γ-tocotrienol, from 85.5 to 244 mg·kg-1 of α-tocopherol and from 69 to 319 mg·kg-1 of α-tocotrienol) (Choi & Lee, 2009; Fernández et al., 2013); therefore, it is considered as an alternative for the development of new products in the food and pharmaceutical industry (Davidov-Pardo & McClements, 2015; Peng et al., 2010).
GSO is chemically unstable and susceptible to deterioration by oxidation due to its high degree of unsaturation, especially when exposed to oxygen, light, moisture and high temperatures (Tonon, Grosso, & Hubinger, 2011). An alternative to protect it and increase its shelf life is microencapsulation, which consists of generating a physical barrier to protect it from environmental conditions (Ahn et al., 2008). During this process a film is formed on the surface of the oil droplet, while the water droplets are evaporated. There are several techniques for the microencapsulation of materials, although, in general, the methods are divided into three types: chemical, physico-chemical and physico-mechanical. Among the last-mentioned is spray drying, which is widely used in the food industry because it has short drying times and great economic potential for scaling up processes (Jyothi et al., 2010; Shishir & Chen, 2017).
There are several reports related to the microencapsulation of oils (fish, rapeseed, canola, orange and mint) where different biopolymers are used as encapsulating agents: whey protein concentrate (Bonilla, Azuara, Beristain, ( Vernon-Carter, 2010), gum arabic, mesquite gum (Rodríduez-Huezo, Pedroza-Islas, Prado-Barragán, Beristaín, & Vernon-Carter, 2004), maltodextrin, inulin (Saénz, Tapia, Chávez, & Robert, 2009), gelatin (Madene, Jacquot, Scher, & Desobry, 2006) and pectin. The last-mentioned is used as wall material in the microencapsulation of bioactive and nutraceutical compounds (Humblet-Hua, Scheltens, van der Linden, ( Sagis, 2011; Polavarapu, Oliver, Ajlouni, ( Augustin, 2011). With proteins and phenolic compounds derived from plant extracts, pectin forms highly protective protein-polyphenol-pectin complexes that generate stable capsules that can be added to functional foods (Faridi, Jafari, ( Assadpour, 2017).
In recent years, the hawthorn (Crataegus spp.) fruit has attracted special attention because it is rich in phenolic compounds, flavonoids and procyanidins. In China, Mexico and several European countries, hawthorn has been widely used as a medicinal material for its health benefits (reduced risk of cardiovascular disease and respiratory problems), for its great potential in the development of functional foods (Huang et al., 2018; Zhu et al., 2013) and for being an important source of pectin (Li, Huang, Dong, Zhu, & Li, 2017; Liu, Kallio, & Yang, 2011).
The genus Crataegus is native to the temperate regions of the northern hemisphere, and is composed of approximately 280 species (Vivar-Vera, Salazar-Montoya, Calva-Calva, & Ramos-Ramírez, 2007; Zhu et al., 2013). Of these, Mexico has about 15 species, most of them native to the central and southern parts of the country, all with phytochemical content associated with the antioxidant activity of hawthorn fruits, although this activity is little known (García-Mateos, Ibarra-Estrada, & Nieto-Ángel, 2013). The germplasm bank of the Universidad Autónoma Chapingo, Mexico, unique in the world, contains 140 accessions of hawthorn (Crataegus spp.) collected from seven regions of Mexico (central and southern parts of the country) (Betancourt-Olvera, Nieto-Ángel, Urbano, ( González-Andrés, 2017; Nieto-Ángel, Pérez-Ortega, Núñez-Colín, Martínez-Solís, & González-Andrés, 2009).
Hawthorn fruits are rich in vitamin C, carotenes and mineral salts, mainly calcium, phosphorus, and iron, and are consumed fresh or processed in products such as cakes, quince pastes, jams, juices, canned foods, liquors and beverages; in addition, they are used in the food industry as a source of pectin, vitamin C and dietary compounds (Edwards, Brown, Talent, Dickinson, & Shipley, 2012; Nieto-Ángel et al., 2009; Vivar-Vera et al., 2007). Zhu et al. (2013) note that pectin isolated from hawthorn fruit has a viscosity four to six times higher than commercial apple and lemon pectins. Due to its characteristics, hawthorn pectin (HP) can be used as a wall material for microencapsulating bioactive compounds, which gives this fruit great economic importance; however, its potential has not been studied. In recent years, studies on hawthorn pectins have been limited to physical-chemical characterization, phenolic compound content, optimization methods, gel formation and emulsion stability (Cuevas-Bernardino, Lobato-Calleros, Román-Guerrero, Álvarez-Ramírez, ( Vernon-Carter, 2016; Linares-García, Ramos-Ramírez, & Salazar-Montoya, 2015; Uysal & Yildirim, 2014).
Recent research related to the encapsulation of bioactive compounds (chia seed oil, fish oil and folic acid) by spray drying reports the use of emulsions and nanoemulsions stabilized with whey protein concentrate, whey protein hydrolysate and commercial citrus pectins (Assadpour, Jafari, & Maghsoudlou, 2017; Noello, Carvalho, Silva, & Hubinger, 2016; Tamm, Härter, Brodkord, & Drusch, 2016). So far, there are no reports on the use of HP-whey protein concentrate (WPC) biopolymer complexes as wall materials for the protection of bioactive compounds. Therefore, the aim of this work was to microencapsulate GSO by spray drying oil/water (O/W) emulsions stabilized with novel biopolymer complexes formed from WPC and HP from two different cultivars, and determine their effect on the morphology and efficiency of microencapsulation.
Materials and methods
The GSO was provided by Vid® (Extractos Naturales Vista al Mar, S.P.R. de R.L., Ensenada, Baja California, Mexico), and the hawthorn (Crataegus spp.) accessions (55 and 100) were obtained from the Germplasm Bank of the Universidad Autónoma Chapingo (Texcoco, State of Mexico, Mexico). In addition, we used WPC (Hilmar 8000, Hilmar Ingredients®, USA, with an isoelectric point of 4.2 ± 0.1, 83.7 % protein and 6.4 % fat), commercial citrus pectin (CP; Cedrosa, Mexico, with a 70 % esterification degree), hydrochloric acid (HCl), sodium hydroxide (NaOH), ammonium hydroxide (NH4OH), petroleum ether and ethyl ether (all analytical grade, Sigma Aldrich®, Mexico). The water used for all experiments was deionized.
Composition of grape seed oil
The fatty acid composition of the GSO was determined in a gas chromatograph (G1530A, Agilent, USA) equipped with an automatic injection turret (model 7683B), a flame ionization detector and a CP-Sil 88 column (100 m × 0.25 mm × 0.39 mm). The initial temperature of the column was 90 °C and increased to 165 °C (20 °C·min-1). The temperature was kept constant for 1 min and then increased to 225 °C (1.5 °C·min-1). The carrier gas was helium with a flow of 0.7 mL·min-1, and methyltridecanoate (C13:0) was used as an internal standard.
Extraction of hawthorn pectin
Pectin was extracted from the accessions (HP55 and HP100) according to the method proposed by Cuevas-Bernardino et al. (2016), with some modifications. First, 100 g of hawthorn fruit pulp were mixed with 1 000 mL of HCl 0.1 N, at 85 ( 1 °C for 60 min and constant stirring. The resulting extract was cooled to room temperature and filtered with Whatman no. 1 paper. Absolute ethanol was then added at a 1:1 (v/v) ratio, and left to stand for 24 h at 4 ( 1 °C. The precipitated pectins were separated by filtration, with the same type of paper, and washed twice with 70 % ethanol at a 1:1 (v/v) ratio, for which the mixture was centrifuged at 1 700 g for 15 min (Sorvall RC-5B, Marshall Scientific, USA). The precipitates were purified with a dialysis membrane (MWCO = 15 000; Spectrum®, USA). Finally, the pectins were dried in a circulating air drying oven (HPP750-230V, Wisconsin Oven Distributors, USA) for 12 h at 30 ( 0.5 °C. The dry-basis HP yield was 4.27 ± 0.32 % and 3.62 ± 0.41 %, for HP55 and HP100, respectively.
Methylesterification degree of hawthorn pectin
Methylesterification degree was determined by the direct titration method described by Jiang et al. (2012), for which CP was used as a reference. First, 500 mg of dehydrated HP and 2 mL of ethanol were dissolved in 100 mL of CO2-free water. The solution was titrated with 0.5 N NaOH, using phenolphthalein as indicator. The volume consumed was established as V A . Subsequently, saponification was performed with 10 mL of NaOH 0.5 N and vigorous stirring; the reaction was carried out for 15 min. Saponification was stopped by adding 10 mL of 0.5 N HCl. The excess HCl was titrated with 0.5 N NaOH, and the consumed volume was established as V B (titration volume B). The esterification degree (ED, %) was calculated from the following equation:
Emulsion preparation
The O/W emulsions were prepared using WPC-CP or WPC-HP (22.2 and 33.3 % [w/w], respectively) in solution as continuous phase and GSO as oily phase. First, 0.3 % sodium azide was added as an antimicrobial agent. The solutions were left to stand for 12 h at 4 ( 1 °C to obtain complete hydration of the biopolymers. A completely random experimental design with three replicates was established to determine the effect of the treatments (Table 1) on the characteristics of the emulsions and microcapsules. The emulsions were made with two total biopolymers:GSO ratios (2:1 and 3:1 w/w) and two concentrations of total solids (TS; 30 and 40 %). The GSO was incorporated dropwise into the biopolymer solutions (WPC-HP55, WPC-HP100 and WPC-CP) with continuous stirring in a homogenizer (Ultra-Turrax T50 Basic, IKA®, USA) operated at 5 200 rpm for 5 min at 30 °C. The emulsions were stored at 4 ( 1 °C until analysis.
Table 1 Composition of emulsions and microcapsules.
| Emulsion (E) | Microcapsules (M) | WPC1-P:GSO ratio | TS (%) |
|---|---|---|---|
| EWPC-CP,2:1 | MWPC-CP,2:1 | 2:1 | 30 |
| EWPC-HP55,2:1 | MWPC-HP55,2:1 | 2:1 | 30 |
| EWPC-HP100,2:1 | MWPC-HP100,2:1 | 2:1 | 30 |
| EWPC-CP,3:1 | MWPC-CP,3:1 | 3:1 | 40 |
| EWPC-HP55,3:1 | MWPC-HP55,3:1 | 3:1 | 40 |
| EWPC-HP100,3:1 | MWPC-HP100,3:1 | 3:1 | 40 |
1WPC = whey protein concentrate; P = pectin; CP = citrus pectin; HP55 = hawthorn pectin from accession 55; HP100 = hawthorn pectin from accession 100; TS = total solids.
Viscosity of emulsions
For viscosity measurements, a Physica MCR 301 dynamic stress rheometer (Physica Messtechnik, Germany) was used, equipped with a cone-plate geometry, in which the rotary cone was 50 mm in diameter and the cone angle 1°. The samples of each emulsion were carefully placed in the measuring system, and the apparent viscosity was determined at 20 °C, for which a shear rate of 10-3 to 103 s-1 was used. The data were plotted on a logarithmic scale of apparent viscosity as a function of the shear rate.
Emulsion droplet size and morphology
The mean surface diameter (d 3,2 ) distribution of the emulsions was determined by a laser diffraction particle size analyzer (Mastersizer 3000, Malvern Instruments Ltd., UK); for this, the emulsion was dispersed in water within the measuring unit (Hydro EV, Malvern Panalytical, UK) until reaching a darkening rate of 10 to 20 %. The refractive indices of GSO (1.468) and water (1.330), as dispersion medium, were determined with a refractometer (44-501, ABBE, USA). The results were reported as d 3,2 , which is defined as:
where z i is the number of droplets with diameter d i .
The emulsion droplets were examined under an optical microscope (BX41, Olympus Optical, Japan) coupled to a digital camera (Infinity 1, Lumenera Corp., Canada) and Image-Pro Plus ver. 7.0 software (Media Cybernetics, USA). Prior to the analysis, the emulsion was diluted in distilled water (1:10 v/v), after which a small sample of the dilution was taken, placed on a glass slide with a coverslip and observed under the microscope.
Preparation of microcapsules
The emulsions were spray-dried (Nichols/Niro, Turbo Spray PLA, USA) at an air inlet and outlet temperature of 170 ( 5 and 85 ( 5 °C, respectively, and atomization pressure at 4 bar (Rodea-González et al., 2012). The emulsions were pumped to the spray dryer at a feed rate of 40 mL·min-1. The dried powders were stored in glass jars wrapped in aluminum foil at 4 ( 1 °C until analysis.
Encapsulation efficiency of grape seed oil
The microencapsulation efficiency (MEE) was calculated from the GSO extractable from the microcapsules with solvent. The free oil was extracted using the method described by Polavarapu et al. (2011). The powder microcapsules (1 g) were added to 10 mL of petroleum ether in a flask; the mixture was stirred in the dark at 25 °C for 15 min and filtered with Whatman paper no. 541. The powder collected in the filter paper was washed three times with 10 mL of petroleum ether; the solvent was then evaporated in a rotary evaporator at 30 °C. The non-encapsulated oil (extractable GSO) was determined by the difference between the initial weight of the bottle without sample and the final weight of the bottle containing the residue of the extracted oil. The total microencapsulated GSO was determined according to the Ross-Gotlieff method (Association of Official Analytical Chemists [AOAC], 1995). The MEE was calculated according to the following equation:
where GSO t is the total oil content and GSO e is the extractable surface oil content.
Particle size distribution
To determine the average diameter of the microcapsules, they were rehydrated in water to obtain reconstituted emulsions (REE), with a total solids content similar to emulsions. The solution was magnetically stirred for 180 min at 70 °C; the REEs were then left to stand at room temperature (20 °C) and the particle size distribution was determined in the same way as with the emulsions.
Scanning electron microscopy
The morphology of the microcapsules was determined using the method proposed by Guadarrama-Lezama et al. (2012). The microcapsules were observed under a scanning electron microscope (JSM-6390/LGS, JEOL, Japan) operated at 10 kV, for which a carbon adhesive tape was placed on the brass sample holder. The samples of the microcapsules were fixed on the sample holder with the aid of a brush and metallized with a thin layer of gold/palladium (80/20) with a metal ionizer (JEOL Fine Coat JFC/1100, JEOL, Japan). The samples were observed under the microscope with magnifications of 500, 2 700, 3 000 and 5 000 x.
Results and discussion
Composition of grape seed oil
The fatty acid composition in the GSO was obtained from the peak area of the chromatograms. The oil used consists of 17 fatty acids, of which the most abundant were: linoleic acid (C18:2), oleic acid (C18:1), palmitic acid (C16: 0) and stearic acid (C18: 0), with 72.3, 14.6, 7.4 and 4.5 %, respectively; the rest had concentrations lower than 2.2 %. The values obtained are very similar to those reported by Bail, Stuebiger, Krist, Unterweger, and Buchbauer (2008) and Coelho et al. (2018).
Esterification degree of hawthorn pectin
The EG of HP55 and HP100 was 70.25 ± 0.90 and 61.01 ± 0.19 %, respectively, indicating that more than 50 % of the pectin carboxyl groups were esterified; therefore, the HP of both accessions was classified as high methoxyl pectin. These results are relevant because pectins with different degrees of methoxylation can be obtained from hawthorn, possibly providing them with different functionality. There are reports in which it is stated that the carboxylic groups of the galacturonic acid chain of pectins can be more or less esterified with methoxyl groups depending on the botanical origin, the location of the pectin (cell wall or middle lamella), the climate and the type of extraction process (Fissore, Rojas, & Gerschenson, 2012; Joye & Luzio, 2000).
Viscosity of grape seed oil emulsions
The apparent viscosity versus shear rate curves of the emulsions (Figure 1) indicated that all emulsions exhibited a similar viscous response, which was associated with a typical pseudoplastic shear-thinning behavior, characteristic of flocculated emulsions and polymeric fluids (Rodea-González et al., 2012). The application of shear forces modifies the macromolecular organization and the rupture of structural units by the action of hydrodynamic forces (Rao, 2007). The curves show a Newtonian behavior at a low shear rate, in which there are ruptures and formation of bonds within the network in a balanced manner, so that there is no net change in molecular organization, and the apparent viscosity remains constant. At high shear rates, bond rupture predominates over the formation of new structures, as molecules align in the direction of flow and apparent viscosity decreases with increasing shear rates (Sittikijyothin, Torres, & Gonçalves, 2005).
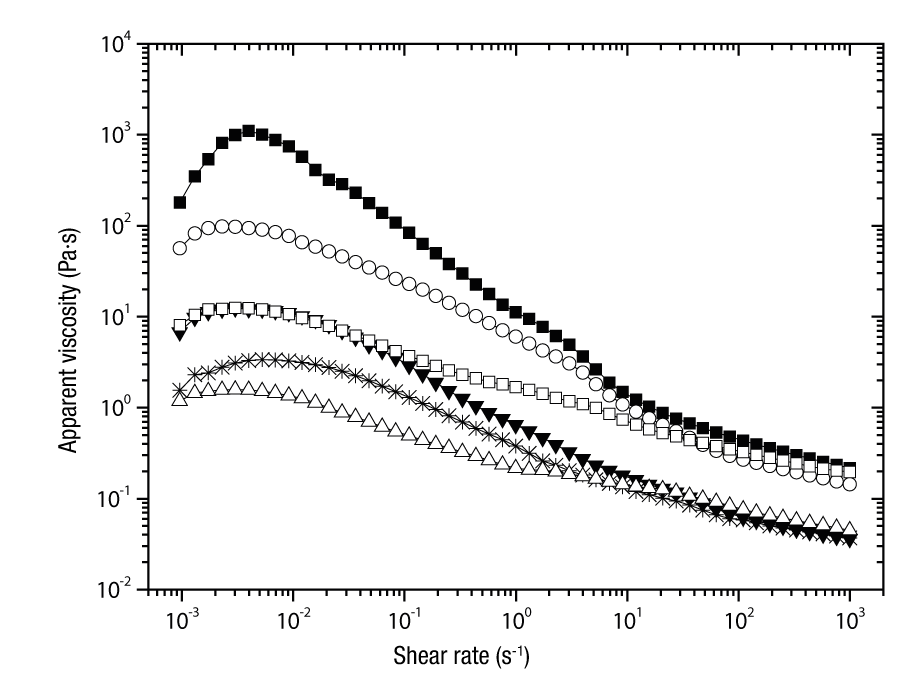
Figure 1 Apparent viscosity against the shear rate of grape seed oil emulsions (30 and 40 % total solids), prior to spray drying: EWPC-CP,3:1 (□); EWPC-HP55,3:1 (■); EWPC-HP100,3:1 (○); EWPC-CP,2:1 (Δ); EWPC-HP55,2:1 (▼); EWPC-HP100,2:1 (*). E = emulsion; WPC = whey protein concentrate; CP = citrus pectin; HP55 = hawthorn pectin from accession 55; HP100 = hawthorn pectin from accession 100; 3:1 = ratio of total biopolymers:oil (w/w); 2:1 = ratio of total biopolymers:oil (w/w).
As expected, emulsions with higher TS content (40 %) had higher viscosity values across the shear rate range than those containing 30 %. The EWPC-HP55,3:1 treatment had the highest apparent viscosity, while the rest had viscosities in the following order: EWPC-HP100,3:1 > EWPC-CP,3:1 > EWPC-HP55,2:1 > EWPC-HP100,2:1 > EWPC-CP,2:1. These results are similar to those reported by Rodríguez-Huezo et al. (2004) in multiple emulsions, where the higher the solids content, the greater the apparent viscosities, regardless of the biopolymer mixture used.
The variation of apparent viscosity against the emulsion shear rate can be predicted by the Carreau model (Equation 4), which has been used for fitting gel flow curves. The original model contains four parameters: η 0 , η ∞ , λ and N (Sittikijyothin et al., 2005).
where η
∞
is the viscosity at high shear rates (Pa·s), η
0
is the initial viscosity at zero shear rate (Pa·s), λ is
a time constant related to the relaxation time (s),
The emulsions showed significant differences in the parameter values of the modified Carreau model (Table 2). These results indicate that the amount of total solids in the emulsions caused the formation of different structural arrangements related to the macromolecules and the dispersed phase. Relaxation times (λ) are directly related to the rupture and recovery time of system linkages. The EWPC-CP,2:1 treatment was the one with the significantly (P < 0.05) lower λ value, and EWPC-HP5,3:1 had the highest λ value. By decreasing λ, the emulsion requires less time and less energy for the flocs to be disintegrated (breakdown of interactions) or smaller flocs to form. As the shear rate increases, the droplets become deformed and the interactions between them are lost. Logaraj, Bhattacharya, Udaya-Sankar, and Venkateswaran (2008) established that the action of the shear rate during the measurement of the rheological properties mainly affects the weak forces or weak interparticle interactions (hydrogen bridges and van der Waals forces), which allows a continuous deformation, where the viscosity decreases progressively.
Table 2 Parameter values of the modified Carreau model of emulsions after storage at 20 °C for 1 day.
| Emulsion code | λ1 (s) | η0 (Pa·s) | N (dimensionless) |
|---|---|---|---|
| EWPC-CP,2:1 | 14.54 ± 0.22 ez | 1.55 ± 0.32 e | 0.15 ± 0.02 e |
| EWPC-CP,3:1 | 93.35 ± 0.61 c | 10.44 ± 0.21 c | 0.17 ± 0.02 e |
| EWPC-HP55,2:1 | 127.32 ± 2.14 b | 10.30 ± 0.71 c | 0.26 ± 0.01 c |
| EWPC-HP55,3:1 | 145.50 ± 4.41 a | 956.53 ± 35.7 a | 0.35 ± 0.02 a |
| EWPC-HP100,2:1 | 35.68 ± 0.48 d | 3.85 ± 0.17 d | 0.21 ± 0.01 d |
| EWPC-HP100,3:1 | 127.81 ± 2.99 b | 102.71 ± 0.52 b | 0.30 ± 0.01 b |
1λ = relaxation times; η0 = initial viscosity at zero shear; N = flow behavior index. zMeans with the same letter within each column do not differ statistically (Fisher, P ≤ 0.05). Mean values ± standard deviation.
Parameter N is a dimensionless exponent that is related to the flow behavior index and reflects how close the emulsions come to a Newtonian behavior. If N = 1 the system behaves as a Newtonian fluid, N < 1 the fluid presents a shear-thinning behavior and N > 1 the fluid shows a shear-thickening behavior (Erçelebi & Ibanoglu, 2009; Rao, 2007). In this case, all emulsions had N values lower than 1; that is, they presented a pseudoplastic shear-thinning behavior, which can be considered as the result of structural modifications in the macromolecular network. This means that by increasing the shear rate a greater number of intermolecular bonds and interactions are broken, with respect to the formation of new bonds. The applied stress forces the molecules to align in the direction of flow and the apparent viscosity decreases (Sittikijyothin et al., 2005). Similar results were reported by Logaraj et al. (2008) in avocado and watermelon oil emulsions, which behaved as non-Newtonian liquids, as they had shear-thinning characteristics with N values from 0.86 to 0.88 (Gharsallaoui et al., 2010).
Emulsion droplet size and morphology
In the droplet size distribution of the emulsions, two perfectly differentiated groups were detected (Figure 2). The group formed by EWPC-CP,3:1, EWPC-HP55,3:1 and EWPC-HP100,3:1 (all with 40 % TS) exhibited a unimodal distribution, while the second group formed by EWPC-CP,2:1, EWPC-HP55,2:1 and EWPC-HP100,2:1 (with 30 % TS) had a bimodal distribution. The treatments with the largest droplet diameters were EWPC-HP100,2:1 and EWPC-HP55,2:1, as a result of the aggregation of the oil droplets. The microscopic analysis (Figure 3) indicated that the large diameters result from the presence of large oil droplets generated by flocculation, which increases as the TS and pectin content decreases; in addition, the formation of a simple O/W emulsion was confirmed, composed of small spherical droplets of oil distributed individually in the continuous aqueous phase (Figure 3).
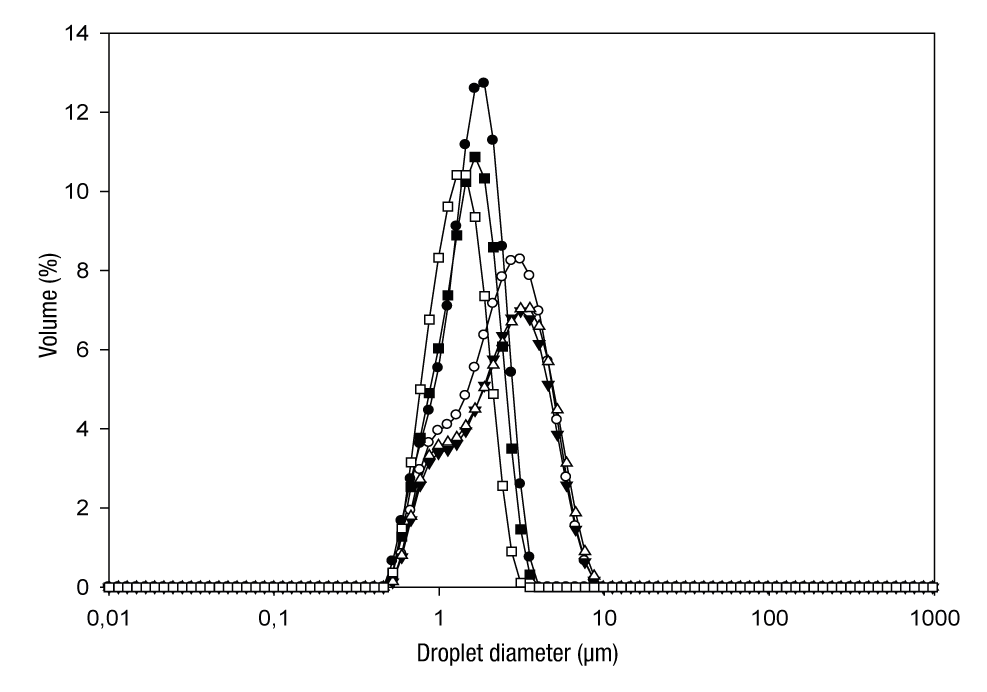
Figure 2. Droplet size distribution of grape seed oil emulsions (30 and 40% total solids) prior to spray drying: EWPC-CP,3:1 (●); EWPC-HP55,3:1 (■); EWPC-HP100,3:1 (□); EWPC-CP,2:1 (○); EWPC-HP55,2:1 (Δ); EWPC-HP100,2:1 (▼). E = emulsion; WPC = whey protein concentrate; CP = citrus pectin; HP55 = hawthorn pectin from accession 55; HP100 = hawthorn pectin from accession 100; 3:1 = ratio of total biopolymers:oil (w/w); 2:1 = ratio of total biopolymers:oil (w/w).
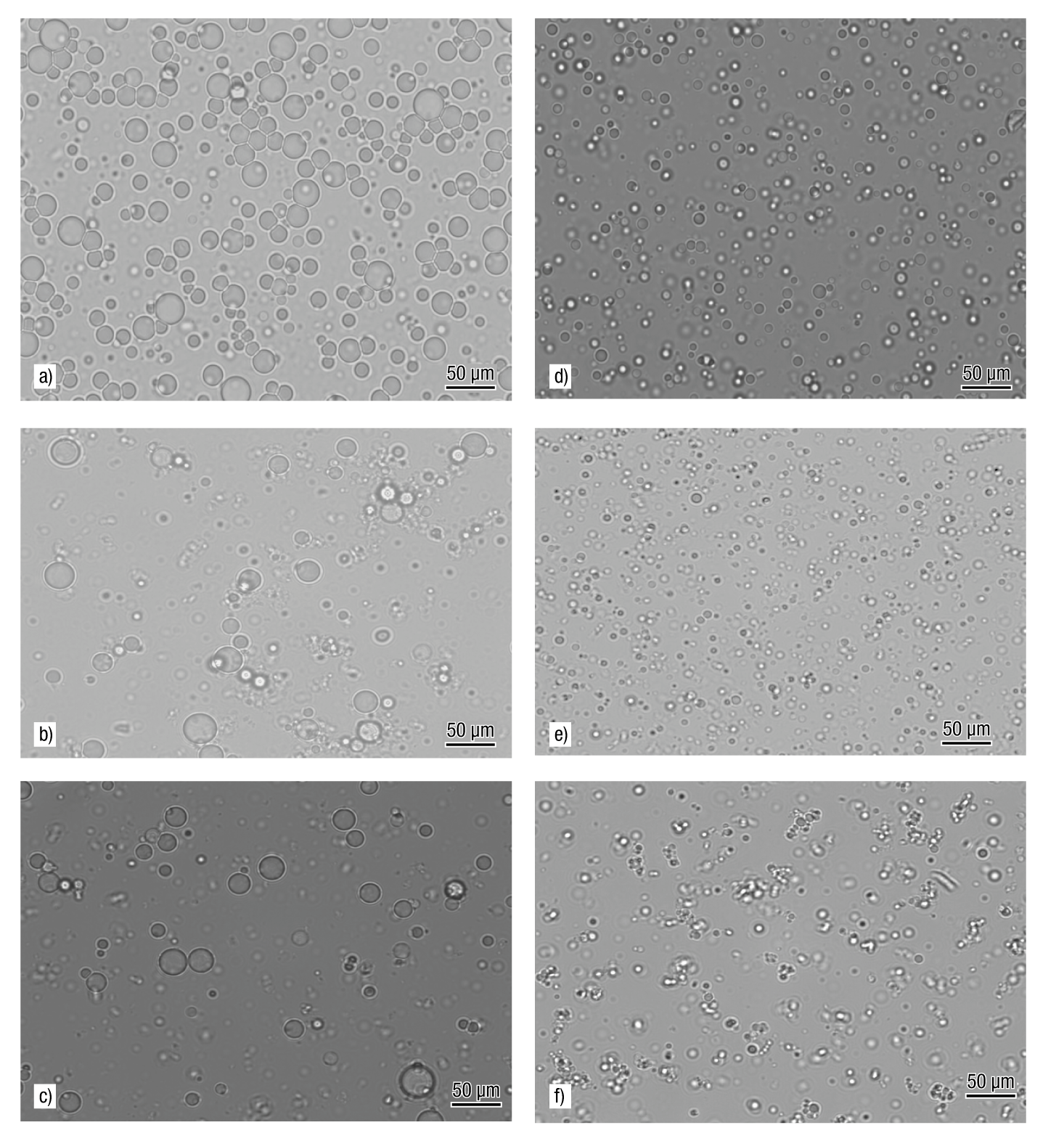
Figure 3 Optical micrographs of oil-in-water emulsions after 1 day of storage: a) EWPC-CP,2:1, b) EWPC-HP55,2:1, c) EWPC-HP100,2:1, d) EWPC-CP,3:1, e) EWPC-HP55,3:1 and f) EWPC-HP100,3:1. E = emulsion; WPC = whey protein concentrate; CP = citrus pectin; HP55 = hawthorn pectin from accession 55; HP100 = hawthorn pectin from accession 100; 3:1 = ratio of total biopolymers:oil (w/w); 2:1 = ratio of total biopolymers:oil (w/w).
The EWPC-HP100,3:1 treatment had the lowest d 3,2 value (1.45 μm). Small droplet diameter values may be due to a combined effect of the high availability of the emulsifier, as it favors a larger oil droplet surface area to be covered with the protein-pectin complex and therefore generates a strong electrostatic repulsion between drops (Neirynck, van der Meeren, Lukaszewicz-Lausecker, Verbeken, & Dewettinck, 2007). The type of pectin added affected the d 3,2 value, this considering that the EWPC-HP55,3:1 droplets were larger than the EWPC-HP100,3:1 ones. This could be related to the fact that HP55 had a higher ED (70.25 %) than HP100 (61.01 %), since the latter contains a higher number of -COOH groups in its chain and generates a higher number of interactions with the protein, which favors that a higher amount of emulsifier is present on the surface of the oil droplets.
The results also indicated that the viscosity of the emulsion had an effect on droplet diameter. The EWPC-CP,2:1 treatment had the largest droplet size, lowest viscosity and lowest TS content. The droplet size of the emulsion depends on the concentration of biopolymers in the aqueous phase, since the higher the concentration of biopolymer mixtures, the higher the viscosity and the smaller the size of the droplets (Rodríduez-Huezo et al., 2004). Carneiro, Tonon, Grosso, and Hubinger (2013) reported the same behavior in the droplet size of their emulsions, indicating that the higher the viscosity, the greater the resistance of the droplets to movement, which avoids coalescence and results in smaller droplets. Generally, reducing the droplet size of the emulsion represents an increase in stability and retention of active material. Gharsallaoui et al. (2010) found that oil droplets covered by protein-pectin complexes showed better emulsion stability than those covered only with protein; this stabilizing effect of pectin could be attributed to steric repulsion.
Encapsulation efficiency
The main requirement for a wall material in microencapsulation is that it has a good ability to retain and seal the core material during processing and storage (Jiménez, García, & Beristain, 2006). The microcapsules had a MEE of 64.97 to 71.29 % and, according to Table 3, the MEE of the GSO was significantly (P < 0.05) influenced by the type of pectin used and the amount of TS (30 to 40 %), with the best being those made with CP and with HP100, both with high solids content. In other words, emulsions with higher TS content, higher viscosity values and smaller droplet diameters presented the highest MEE. Likewise, it was observed that the most stable emulsion was the one that had the highest MEE, which coincides with what was reported by Carneiro et al. (2013). These authors also note that MEE may be related to droplet sizes and emulsion viscosities, which depends on the botanical origin of the pectin used as wall material.
Table 3 Mean surface diameter and microencapsulation efficiency of reconstituted grape seed oil microcapsules.
| Microcapsules | d3,2 (μm) | MEE (%) |
| 1MWPC-CP 2:1 | 1.84 ± 0.01 bz | 66.84 ± 0.98 c |
| MWPC-CP 3:1 | 2.23 ± 0.01 d | 70.33 ± 1.09 ab |
| MWPC-HP55 2:1 | 2.59 ± 0.01 e | 64.97 ± 0.80 c |
| MWPC-HP55 3:1 | 1.60 ± 0.01 a | 69.13 ± 0.91 b |
| MWPC-HP100 2:1 | 2.08 ± 0.02 c | 65.92 ± 0.97 c |
| MWPC-HP100 3:1 | 1.86 ± 0.01 b | 71.29 ± 0.14 a |
1M = microcapsule; d 3,2 = mean surface diameter; MEE = microencapsulation efficiency; WPC = whey protein concentrate; CP = citrus pectin; HP55 = hawthorn pectin from accession 55; HP100 = hawthorn pectin from accession 100; 3:1 = ratio of total biopolymers:oil (w/w); 2:1 = ratio of total biopolymers:oil (w/w). zMeans with the same letter within each column do not differ statistically (Fisher, P ≤ 0.05). Mean values ± standard deviation.
Rodea-González et al. (2012) reported similar MEE values for chia essential oil with WPC-polysaccharide matrices. On the other hand, Lim, Tan, Bakar, and Ng (2011) obtained an efficiency of 77.61 to 85.3 % using different wall materials to microencapsulate pitaya seed (Hylocereus polyrhizus) oil. Considering both works, it can be said that the values obtained for GSO are within the reported range. Tonon et al. (2011) indicate that treatments with higher solids content showed higher MEE, which were associated with higher emulsion viscosities and smaller droplet size.
Micromorphology of microcapsules
Figure 4 shows the SEM micrographs of the powders produced with different combinations of wall material. All microcapsules showed a similar external topography, characterized by rounded shapes and a continuous wall with no apparent cracks or fissures on the outer surface, providing lower gas permeability and better protection and retention of the GSO. In addition, shriveled areas, with depressions or hollows, can be seen on the outer surface, a typical characteristic of particles produced by spray drying (Carneiro et al., 2013). The presence of voids is common in drying processes, and is related to the formation of vacuoles within the solid crust of the particle. When the temperature reaches the boiling point of water, the crust expands and traps water vapor and air along with the active material (Tonon et al., 2011). This expansion process is associated with the composition and viscosity of the emulsion fed to the dryer (Gharsallaoui et al., 2010).
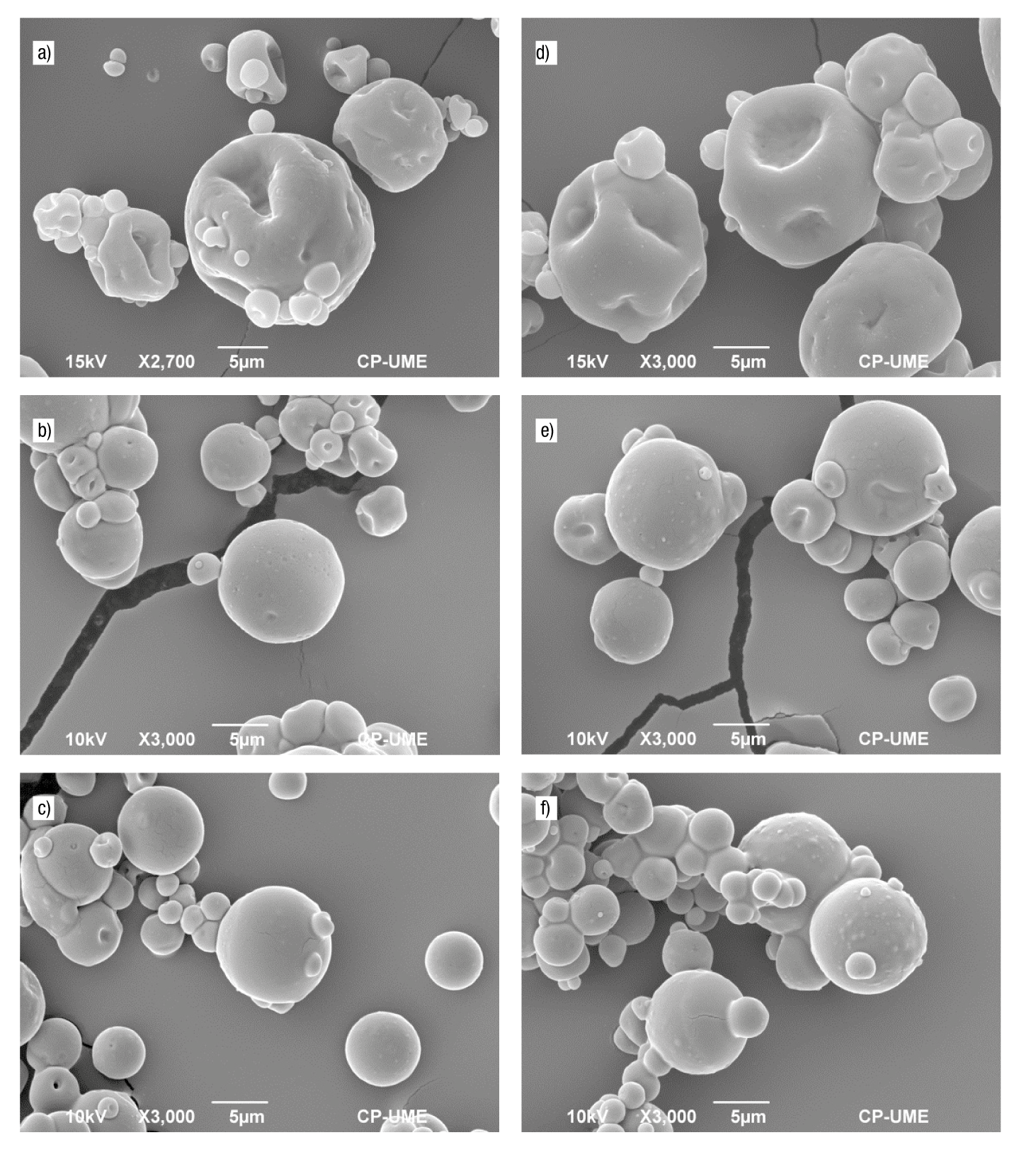
Figure 4 Micrographs of grape seed oil microcapsules: a) MWPC-CP,2:1, b) MWPC-HP55,2:1, c) MWPC-HP100,2:1, d) MWPC-CP,3:1, e) MWPC-HP55,3:1 and f) MWPC-HP100,3:1. M = microcapsule; WPC = whey protein concentrate; CP = citrus pectin; HP55 = hawthorn pectin from accession 55; HP100 = hawthorn pectin from accession 100; 3:1 = ratio of total biopolymers:oil (w/w); 2:1 = ratio of total biopolymers:oil (w/w).
Microcapsules made with CP (MWPC-CP,2:1 and MWPC-CP,3:1) showed larger depressions on the outer surface than those made with HP. On the other hand, microcapsules made with EWPC-HP100,3:1 showed smaller depressions because this emulsion had higher apparent viscosity and lower d 3,2 values. This behavior is similar to that obtained by Rodríguez-Huezo et al. (2004) in carotenoid microencapsulates when using multiple emulsions with different solids contents. Other researchers reported morphological characteristics similar to those obtained by spray drying in this work, also in microcapsules (Favaro-Trindade, Santana, Monterrrey-Quintero, Trindale, & Netto, 2010; Loksuwan, 2007; Rodea-González et al., 2012).
Conclusions
The use of O/W emulsions with well-functioning wall materials, such as whey protein and pectin from different origins, allows stabilization and protection of bioactive ingredients. The HP-protein interaction allowed the formation of thicker physical barriers, with high MEE and adequate morphology, which can stabilize GSO against oxidation processes. The GSO’s MEE was influenced by the TS content and the type of pectin used. The emulsions with hawthorn pectin from accessions 55 and 100, with 40 % TS, had the highest viscosities in the whole shear rate range. The EWPC-HP100,3:1 treatment produced microcapsules with the highest MEE (71.29 %) and the smallest emulsion droplet diameter (d3,2 = 1.45 μm). Generally, a reduction in droplet size is associated with greater stability for possible use in food matrices. The morphology of the capsules was affected by the type of biopolymer and the concentration of the wall materials. Microcapsules with HP100 had spherical particles with smaller dents on the outer surface than those formulated with CP.











 texto en
texto en 


