Introduction
The water hyacinth (Eichhornia crassipes) is a floating tropical species native to Brazil and, possibly, some Central American countries. It shows rapid growth and its distribution has been reported in several parts of the world, mainly between 40° North and South latitude, including South Africa and some countries such as India and the United States (Center, 1994; Hellmann, Byers, Bierwagen, & Dukes, 2008). In its natural habitat, the water hyacinth plays a very important role because it provides shelter and feeding grounds for fish, phytoplankton and zooplankton (Villamagna & Murphy, 2010), and it also has characteristics with biotechnological potential. However, in large quantities, this species has negative effects on the environment, human health and the economy, since it directly affects aquatic life in freshwater bodies (Epstein, 1998) and there is no biological control to prevent its excessive proliferation (Humphrey & Dube, 2018).
This plant is capable of developing in waters with high nutrient content, since it tolerates large variations in nutrients, pH and temperature (between 1 and 40 °C), although its optimum pH is between 6 and 8 (Wilson, Holst, & Rees, 2005). The growth rate of the water hyacinth increases when there are high levels of nitrogen dissolved in the water (Heard & Winterton, 2000), and it has been observed that salinity levels of 6 to 8 % are lethal to the plant (Olivares & Colonnello, 2000).
One of the main problems generated by the water hyacinth is that, due to its high growth rate, it quickly covers the surfaces of water bodies, which prevents the passage of light and, consequently, blocks the photosynthetic processes of the aquatic flora, thereby reducing the dissolved oxygen in the water. In addition, its antagonistic role in the growth of other aquatic organisms, which reduces biological diversity, has been described (Brendonck et al., 2003). This species is capable of doubling its population in just five days, and has reached up to two million plants per hectare, weighing 200 to 400 t, which also generates serious problems in terms of navigation, recreation, irrigation and electricity generation (Epstein, 1998).
For the control of the water hyacinth, mathematical growth models have been developed, and it has been observed that under controlled conditions its growth follows a logistic behavior (Wilson et al., 2005); however, this has not been enough to avoid all the problems it causes in areas where it is considered an invasive species. Chemical methods (herbicides), biological control agents and physical removal are currently being used to control its growth (Harley, Julien, & Wright, 1996). It is a fact that only one method is inefficient, so it is necessary to use several removal techniques simultaneously (Gutiérrez, Ruiz, Uribe, & Martínez, 2000), hence the importance of generating processing alternatives.
The water hyacinth contains between 92.8 and 95 % water, 4.2 to 6.1 % volatile compounds, 18.2 to 19 % cellulose, 48.7 to 50 % hemicellulose, 3.5 to 3.8 % lignin and 13 to 13.5 % crude protein (Nigam, 2002), so it can be used in a wide variety of processes (Malik, 2007); however, it is important to consider that these concentrations may vary depending on the geographical area and climatic conditions (Boyd & Vickers, 1971).
The most common uses of water hyacinth are for the production of ethanol (Aswathy et al., 2010; Guragain, Coninck, Husson, Durand, & Rakshit, 2011; Nigam, 2002), the production of fungal enzymes (Deshpande, Nair, & Khedkar, 2009; Pramanik, 2010; Wang, Liu, Ning, Liao, & Jia, 2017), the removal of heavy metals in water (Mahamadi & Nharingo, 2010; Mishra & Tripathi, 2009; Muramoto & Oki, 1983; Saraswat & Rai, 2010), phytoremediation (Jayaweera & Kasturiarachchi, 2004), biogas production (Kivaisi & Mtila, 1997) and as organic fertilizer (Khaket, Singh, Dhanda, Singh, & Singh, 2012).
Several researchers have analyzed the components of water hyacinth from different geographical areas. Lara-Serrano et al. (2016) studied samples from two geographical areas of Mexico (Durango and Guanajuato), finding differences in ash, holocellulose, and total extractives, while Lakshminarayana, Rao, Pantulu, and Thyagarajan (1984) observed differences in the composition of lipids in roots, stalks, leaves, and flowers. Therefore, in order to generate strategies for the use of this species, it is essential to know the influence that the geographical area and the parts of the plant have on its composition. In this sense, this research aimed to evaluate the effect of the collection area and the section of the Eichhornia crassipes plant on its chemical composition in four geographical zones of Mexico (Mexico City, Hidalgo, Jalisco and Tabasco), in order to determine the presence of the main components of this species for possible biotechnological applications.
Materials and methods
Collection of samples
Samples were collected in four areas from June to August 2016: San Antonio Dam (Huasca de Ocampo, Hidalgo: 20° 13’ 40” NL and 98° 33’ 45” WL), Cuemanco Pier (Mexico City: 19° 16’ 06” NL and 99° 06’ 15” WL), Chapala Lake (Jalisco: 19° 16’ 32” NL and 99° 06’ 05” WL) and Villahermosa (Tabasco: 18° 10’ 99” NL and 93° 10’ 92” WL).
Sample preparation
Fifty specimens were taken per collection area, within which there were mature, medium-aged and young plants; this was done at the same time of year so that the effect of the plant's age on its chemical composition was minimal. The plants were washed with cold water, sectioned and classified: small leaf (SL) and small stalk (SS), medium-sized leaf (ML) and medium-sized stalk (MS), large leaf (LL) and large stem (LS), for the upper, middle and lower parts of the plant, respectively (Figure 1). Samples were dried at 60 °C for 36 h with a convection oven (DHG-9000J, MTI Corporation, USA), then ground, sieved in a 40 mesh (0.425 mm), placed in dark bags and stored in a cool, dry place for analysis.
Chemical analysis
Material extractable in organic solvents. TAPPI standard T-204 om-88 was used by means of a Soxhlet system and hexane as solvent. For the analysis, 10 g of each sample were used.
Material extractable in water. TAPPI standard T-207 om-93 was used in the residue resulting from extraction with solvents.
Ash content. It was determined in accordance with TAPPI standard T-211 om-93, for which 1 g of sample was used.
Lignin insoluble in acid. From 1 g of sample, the percentage of lignin present was determined by weight difference in accordance with TAPPI standard T-222 om-88.
Holocellulose content. One hundred mL of sodium chlorite at 1.5 % and glacial acetic acid were added to 2 g of sample. The reaction was carried out at 75 °C for 5 h. The mixture was then filtered in a Gooch crucible, washed with acetone and dried at 105 °C for 1 h. The percentage of holocellulose was determined by the difference between the initial and final weight.
All samples were analyzed in triplicate and the results were expressed as a percentage on a dry basis.
Results and discussion
To identify significant differences among the parts, a multivariate statistical analysis was carried out (Table 1), which indicated that both the parts (leaves and stalks) and the collection areas (four states of Mexico) had a significant effect on the variables evaluated. On the other hand, in the results of the LSD analysis, it can be seen that the percentage of ash, solvent extractives and water extractives (Figure 2) showed a greater statistical difference (α ≤ 0.05) in the samples from Jalisco.
Table 1 Multivariate test of significance for the analyzed parts (leaves and stalks) and states of Mexico (Mexico City, Hidalgo, Jalisco and Tabasco).
| Value | F | Effect | Error | P | |
|---|---|---|---|---|---|
| Intercept | 0.000051 | 171652.4 | 5 | 44.0000 | 0.00 |
| State | 0.000011 | 494.6 | 15 | 121.8660 | 0.00 |
| Part | 0.000446 | 46.0 | 25 | 164.9547 | 0.00 |
| State x part | 0.000051 | 19.7 | 75 | 214.9682 | 0.00 |
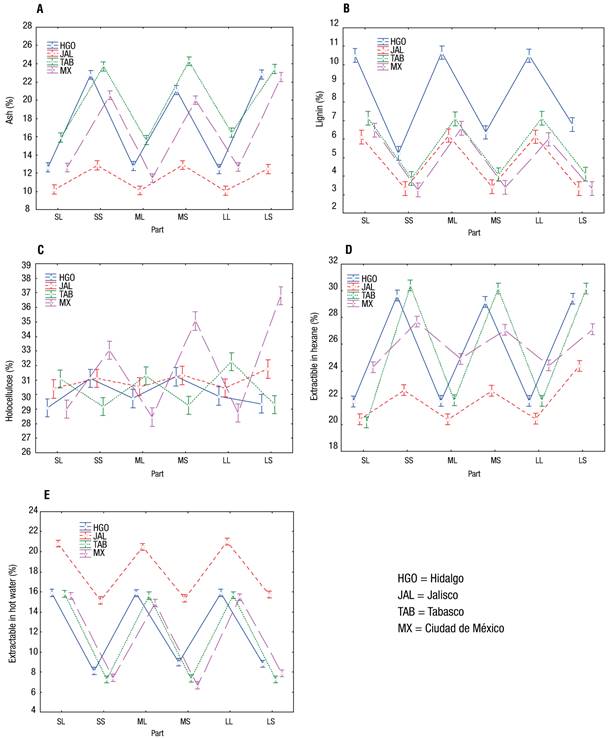
Figure 2 Graphs of the least significant difference (LSD) analysis of water hyacinth samples. SL = small leaf; SS = small stalk; ML = medium-sized leaf; MS = medium-sized stalk; LL = large leaf; LS = large stalk.
Regarding chemical composition, great differences were observed, both in the parts analyzed and in the collection area, with the holocellulose content (cellulose + hemicellulose) having the least variation (Figure 3C). As for ash content, samples from Mexico City, Hidalgo and Tabasco averaged 16.16, 17.56 and 19.8 %, respectively (Figure 3), and showed no statistically significant differences among them (Figure 2). The samples with the lowest concentration were those from Jalisco, while those from Tabasco had the highest (Figure 3A). Gao, Chen, Yuan, Huang, and Yan (2013) report an ash content of 45 % and Ma et al. (2010) of 2.8 %, both of hyacinth collected in China, but from different areas, which could evidence the effect of geographical area on the chemical composition of the plant.
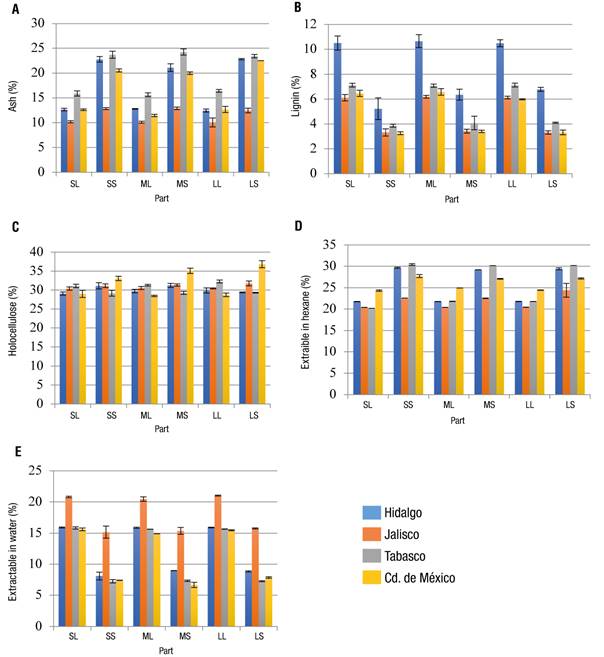
Figure 3 Concentration of the main components of the water hyacinth. SL = small leaf; SS = small stalk; ML = medium-sized leaf; MS = medium-sized stalk; LL = large leaf; LS = large stalk.
Atehortua and Gartner (2013) mention that that the increased ash content may be due to a higher adsorption of metals present in the water, which remain stable during calcination; in this sense, this increase could be an indication of pollution of the waterbodies at the hyacinth collection site. Lara-Serrano et al. (2016) found large differences in the ash content of water hyacinth from two geographical areas of Mexico (Durango and Guanajuato), being higher in the samples from Durango (26 %) than in those from Guanajuato (14.6 %), probably due to different concentrations of elements such as silicon, magnesium, chlorides, potassium, calcium and iron. In general, Figure 3A shows that the stalk has more ash because it functions as a vascular system that allows the flow of nutrients to the leaves and vice versa; also, metals in water are likely to be more easily absorbed by this system.
On the other hand, the highest percentage of lignin was observed in the samples collected in the state of Hidalgo, on average 8 % (Figure 3B), and in all collection areas the highest percentage was found in the leaves. This coincides with Balasubramanian, Arunachalam, Da, and Arunachalam (2012), who found higher lignin content in water hyacinth leaves than roots collected in three geographical areas of India. Lignin plays a very important role since, in addition to protecting the plant against attack by various organisms, it is part of the vascular and support systems of the plant; therefore, since the hyacinth is a surface plant where the leaves are responsible for its buoyancy, it is assumed that the lignin is present in greater quantity in this part of the plant.
Neiff, Casco, and de Neiff (2008) determined that the amount of lignin in E. crassipes is a function of the water levels in the lakes where it grows, and that long periods of confinement of the plant cause an increase in the lignin concentration. It is likely that in the state of Hidalgo there is little water exchange and with low levels, which would explain, in part, the high percentages of lignin found in the hyacinth collected in this state. On the other hand, the hyacinth from Jalisco and Mexico City had the lowest lignin concentrations, on average 4.6 and 4.7 %, respectively (Figure 3), values very similar to those described by Nigam (2002), Sornvoraweat and Kongkiattikajorn (2010), and below those reported by Gao et al. (2013) (17 % lignin in hyacinth from the Liuxi River in China) and Ma et al. (2010) (29.3 % lignin from hyacinth collected in China).
In general, the largest amount of solvent extractives was presented in the stalks of all samples (Figure 3D), with the Jalisco samples having the largest statistical difference (Figure 2). The results obtained agree with the findings of Silva, de Melo, Silvestre, and Silva (2015), who reported in E. crassipes a concentration of solvent extractives of 31.49 and 24.23 % for stalks and leaves, respectively, although they are lower than those reported by Lakshminarayana et al. (1984) (64.4 % of solvent extractives in leaves and stalks). The main extractable compounds can be waxes, resins, fatty acids and their esters, unsaponifiable substances and chlorophyll ( Sluiter et al., 2005). It has been reported that this type of compound can act as a catalyst in the oxidation of lignocellulose, specifically hemicellulose and lignin (Burgois, Bartholin, & Gutonnet, 1989), and that the age of the plant has an important effect on its concentration.
In the case of water-extractable compounds, Figure 3E shows that the greatest amount was presented in the leaves (Figure 3E). In this process, water-soluble compounds such as non-structural carbohydrates, salts and some nitrogenous substances are extracted. The samples collected in Jalisco had the highest percentage of aqueous extractives, with an average value of 20 % in leaves and 15 % in stalks, which is consistent with what was reported by Tan et al. (2008). These results suggest that there is an effect of both the collection area and the plant part.
Finally, the holocellulose content was very similar in all parts, although it was slightly higher in samples collected in Mexico City (Figure 3C). Carbohydrates present in holocellulose are of great interest, as they can be used in a variety of biotechnological processes such as bioethanol production (Guragain et al., 2011). The holocellulose concentration found (about 32 %) differs from that described by other authors, which may be due to differences in climate, plant age, elevation and geographical area, among other things.
Balasubramanian et al. (2012) observed that the amount of holocellulose in water hyacinth collected in three different waterbodies in India was not consistent because it ranged from 68.8 to 78.6 %; furthermore, they found that this percentage was affected by the part of the plant (roots, petioles or leaves). The holocellulose contents reported by other authors in E. crassipes samples, obtained from different geographical areas, were: 53.3 % (Abraham & Kurup, 1996), 66.9 % (Nigam, 2002), 52.9 % (Gunnarsson & Petersen, 2007), 67.6 % (Kumar, Singh, & Ghosh, 2009), 51.7 % (Sornvoraweat & Kongkiattikajorn, 2010), 47.5 % (Ma et al., 2010) and 41.6 % (Gao et al., 2013).
On the other hand, Table 2 shows the results of the contrast analysis (comparison between means), where it can be observed that the State-Part relationship did not present a significant difference; that is, both the stalks and the leaves (parts) of the plants collected in the same state do not have significant differences. The rest of the comparisons showed differences between them. As an example, the State-Ash relationship implies that the ash content of the plants is different in each of the states. In general, the contrast analysis indicated that the parts (leaf and stalk), the components (ash, water extractives, solvent extractives and holocellulose content) and the state (Mexico City, Hidalgo, Jalisco and Tabasco) all show statistical differences when compared to each other.
Table 2 Contrast analysis between the different parts studied (α ≤ 0.05).
| Contrast | Significance | Difference | +/- Limits |
|---|---|---|---|
| State - Part | - | -1.0 | 1.06071 |
| State - Ash | * | -13.8471 | 1.06071 |
| State - Lignin | * | -3.37039 | 1.06071 |
| State - Holocellulose | * | -28.3097 | 1.06071 |
| State - SE1 | * | -22.2604 | 1.06071 |
| State - WE | * | -10.792 | 1.06071 |
| Part - Ash | * | -12.8471 | 1.06071 |
| Part - Lignin | * | -2.37039 | 1.06071 |
| Part - Holocellulose | * | -27.3097 | 1.06071 |
| Part - SE | * | -21.2604 | 1.06071 |
| Part - WE | * | -9.79203 | 1.06071 |
| Ash - Lignin | * | 10.4767 | 1.06071 |
| Ash - Holocellulose | * | -14.4626 | 1.06071 |
| Ash - SE | * | -8.41326 | 1.06071 |
| Ash - WE | * | 3.05508 | 1.06071 |
| Lignin - Holocellulose | * | -24.9393 | 1.06071 |
| Lignin - SE | * | -18.89 | 1.06071 |
| Lignin - WE | * | -7.42164 | 1.06071 |
| Holocellulose - SE | * | 6.04934 | 1.06071 |
| Holocellulose - WE | * | 17.5177 | 1.06071 |
| SE - WE | * | 11.4683 | 1.06071 |
1SE = solvent-extractable compounds; WE = water-extractable compounds.
Due to the variability observed among the samples and to determine if the collection area influences the composition of the plant, homogeneous groups were formed from the LSD analysis (Table 3). It can be seen that in the ash content, the samples from Jalisco, which had the least amount of this compound, did not show differences with respect to the samples from Mexico City in the ML part. In the case of lignin, the largest amount was detected in the samples from Hidalgo, a state that did not form groups in any part, while the lignin present in SS, MS and LS does not differ statistically from each other, since the same groups are formed.
Table 3 Formation of homogeneous groups from the least significant difference (LSD) analysis of the samples from four areas of Mexico.
| Chemical compound | Homogeneous groups (α ≤ 0.05) | |||||
|---|---|---|---|---|---|---|
| SL1 | SS | ML | MS | LL | LS | |
| Ash | Tab Hgo-Mx Jal | Tab-Hgo Mx Jal | Tab Hgo Mx-Jal | Tab Hgo-Mx Jal | Tab Hgo-Mx Jal | Tab-Hgo-Mx Jal |
| Lignin | Hgo Tab-Mx Mx-Jal | Hgo Tab-Jal-Mx | Hgo Tab-Mx Mx-Jal | Hgo Tab-Jal-Mx | Hgo Tab Jal-Mx | Hgo Tab-Jal-Mx |
| Holocellulose | Tab-Jal-Hgo Hgo-Mx | Mx Hgo-Jal Tab | Tab-Jal-Hgo Mx | Mx Hgo-Jal Tab | Tab Jal-Hgo Hgo-Mx | Mx Jal Hgo-Tab |
| Solvent-extractible | Mx Hgo Jal-Tab | Tab-Hgo Mx Jal | Mx Hgo-Tab Jal | Tab-Hgo Mx Jal | Mx Hgo-Tab Jal | Tab-Hog Mx Jal |
| Water-extractible | Jal Hgo-Tab-Mx | Jal Hgo-Mx-Tab | Jal Hgo-Tab Tab-Mx | Jal Hgo Tab-Mx | Jal Hgo-Tab-Mx | Jal Hgo Mx-Tab |
1SL = small leaf; SS = small stalk; ML = medium-sized leaf; MS = medium-sized stalk; LL = large leaf; LS = large stalk; Mx = Mexico City; Hgo = Hidalgo; Jal = Jalisco; Tab = Tabasco.
The holocellulose concentration showed greater heterogeneity, both in stalks and leaves, where the SS, MS and LS samples from Mexico City were grouped independently. The same occurred with the solvent-extractable compounds, of which the samples from Mexico City were independent from the other states, these for the case of SL, ML and LL, and the samples from Jalisco were the ones that showed the greatest differences, except for SL (Table 3). Finally, it was observed in the water-extractable compounds that the Jalisco samples had the highest concentration of these compounds, and showed no similarity with the samples from the other states.
In general, the results obtained can facilitate decision-making regarding the processing of water hyacinth for various biotechnological processes, such as obtaining carbohydrates, or as a possible indicator of waterbody pollution.
Conclusions
The results provide information on the chemical composition of the water hyacinth (E. crassipes) from four areas of Mexico (Mexico City, Hidalgo, Jalisco and Tabasco), which showed great heterogeneity. Both leaves and stalks showed significant statistical differences in chemical composition. Samples from Tabasco had the highest concentration of ash, those from Hidalgo the highest concentration of lignin and those from Jalisco the highest percentages of water-extractible compounds. On the other hand, holocellulose showed the highest variability with least significant differences, both at the level of collection area and plant part. This suggests that for the processing of the plant it is important to take into account the geographical area and the part thereof.











 texto en
texto en 



