Introduction
The quality of potato tubers is influenced by a group of physical and chemical attributes which define their end-use, such as potato skin and flesh color, size and shape, as well as dry matter (DM), starch, and reducing sugars (RS) content (Stark & Love, 2003). Dry matter and starch content are the most important characteristics for the industrial processing because they influence the yield, texture, or oil absorption of cooked and fried products, such as chips and French fries. On the other hand, a lower content of RS is required to avoid non-enzymatic browning and to obtain products with acceptable color and flavor, as well as to reduce acrylamide formation (Pedreschi, Moyano, Kaack, & Granby, 2005).
Genetic, abiotic, and biotic factors affect the chemical composition and physical characteristics of potato tubers. Drought, frosts, and hailstorms damage the plant’s foliage, limiting photosynthesis and starch synthesis and accumulation; therefore, tubers with lower specific gravity (SG) are produced (Stark & Love, 2003). Among biotic factors, pests and diseases such as late blight and zebra chip (ZC) are the main causes of reduced tuber quality (Rubio-Covarrubias, Cadena-Hinojosa, & Vázquez-Carrillo, 2013). ZC disease has been considered a new threat for the potato industry; it has been reported to appear in the majority of the potato production regions in Mexico, the U.S., Central America, and New Zealand (Munyaneza, 2015; Rubio-Covarrubias, Cadena-Hinojosa, Prager, Wallis, & Trumble, 2017).
Potato tubers infected by ZC can be rejected for both fresh consumption and for chip production. Infected tubers are characterized by the browning of internal tissues, which upon frying become more pronounced, and chips or French fries become very dark with an undesirable taste, rendering them commercially unacceptable (Munyaneza, 2012, 2015). Recently, increased levels of phenolics, reducing sugars, enzymes, amino acids, and minerals have been reported to be positively associated with ZC infection (Rubio-Covarrubias et al., 2017; Wallis, Chen, & Civerolo, 2012).
Rubio-Covarrubias, Almeyda-León, Cadena-Hinojosa, and Lobato-Sánchez (2011) reported that the symptoms related to ZC disease, such as the internal browning, decreased as the field production altitude increased; even above 3 200 masl the disease symptoms were not significant. These results were related to a decrease in the population of the insect vector (Bactericera cockerelli Sulc), which requires warmer conditions to develop. In Mexico, the Toluca Valley is one of the most important potato production regions where ZC incidence is high (Rubio-Covarrubias et al., 2015).
In countries where ZC incidence is high, breeding programs have worked intensely to develop ZC-resistant genotypes, although to date no variety has been released commercially (Rubio-Covarrubias et al., 2017). Several studies have reported the effects of environmental and growth conditions on tuber composition and quality (Hamouz, Cepl, & Dvorak, 2005; Hamouz, Lachman, Dvorak, Juzl, & Pivec, 2006; Leonel et al., 2017); however, to our knowledge, all these studies have been carried out in regions or under experimental conditions where ZC incidence is inexistent. Therefore, the aim of this work was to evaluate the effect of the genotype-environment interaction on the physicochemical characteristics of tubers and their influence on chip yield, color, and texture.
Materials and methods
Potato genotypes
Four genotypes were evaluated including three clones (Nau, 5-10, 99-39) and a commercial variety (Fianna) (Table 1).
Field experimental conditions
Genotypes were grown in the spring-summer seasons of 2012 and 2013, at two locations: Metepec and Raíces, both located in the State of Mexico, Mexico. Metepec (19° 14’ 35.39’’ North Latitude, 99° 35’ 27.82’’ West Longitude, at altitude of 2 600 m), has soil sandy loam, pH 5.6, and 1.1 % organic matter, and ZC incidence at this location is high (Rubio-Covarrubias et al., 2015). In contrast, Raíces (19° 9’ 59.78’’ North Latitude, 99° 47’ 53.74’’ West Longitude, at altitude of 3 500 m) present soil clay loam, pH 5.1, high phosphorus fixation capacity, and 3.2 % organic matter, and ZC incidence is null (Rubio-Covarrubias et al., 2013).
At both locations, potatoes were grown by following the potato production technology of these regions (Rubio-Covarrubias et al., 2000). Field plots consisted of two furrows 8 m in length, with 90 cm spacing between furrows and 30 cm spacing between plants. Potatoes of each genotype were planted in a complete block design with four replications. In Raíces, in 2012, sowing took place on May 15, the desiccant was applied on September 21, and harvesting was conducted on October 15; on the other hand, in 2013, these activities occurred on May 20, September 23, and October 14. In Metepec, in 2012, these activities were performed on June 11, September 13, and October 4, while in 2013, they were carried out on June 11, September 12, and September 27, respectively. After harvesting, tubers were stored two days at room temperature (25 °C) until analysis.
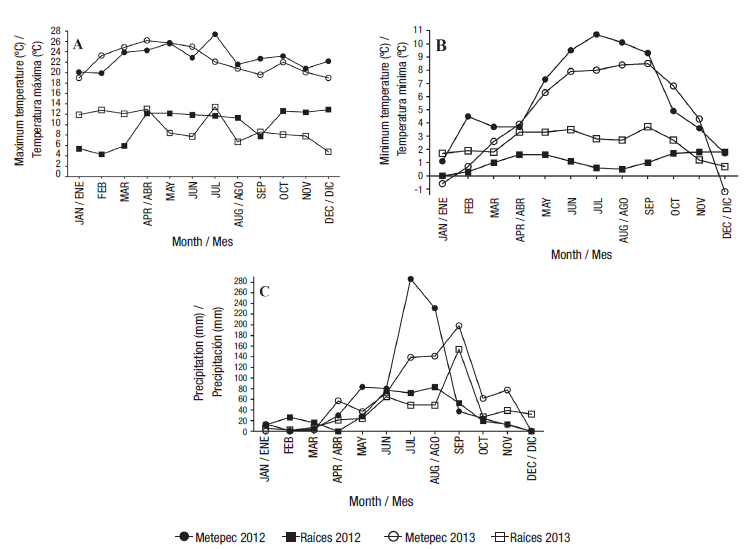
Fertilization doses applied in Raíces were 200-300-200 (N-P2O5-K2O) kg·ha-1, plus 50 kg·ha-1 of micronutrients, and 1 t·ha-1 of chicken manure. In Metepec, the fertilization dosage was 200-200-200 (N-P2O5-K2O) kg·ha-1, plus 50 kg·ha-1 of micronutrients, and 1 t·ha-1 of chicken manure. Fungicides and pesticides were applied as required in order to control pests and diseases. The maximum and minimum temperatures, as well as the precipitation in each location is shown in Figure 1.
Tuber physical properties
Ten tubers of each genotype were analyzed. Internal tuber discoloration was visually scored as reported by Rubio-Covarrubias et al. (2017). Each tuber was cut transversally and the degree of internal browning was graded on the scale from 0-5, where zero indicates no browning and 5 indicates a tuber with severe internal discoloration. Flesh color was evaluated in the cross-section of cut tubers in terms of luminosity (L*), hue, and chroma, using the MiniScan XE Plus colorimeter (Hunter Lab, USA) (Vázquez-Carrillo, Santiago-Ramos, Ybarra-Moncada, Rubio-Covarrubias, & Cadena-Hinojosa, 2013).
The SG was determined by the method described by Gould (1999). Solutions of NaCl (Merck, Germany), with SG between 1.040 and 1.140, were prepared with distilled water at 20 °C. Tubers were placed into 1 L of each solution, starting with the lowest density solution, and then they were moved successively to the following higher-density solution until the solution in which the tuber floated, when SG of this solution was considered the same as that of the tuber. Meanwhile, DM content was determined in triplicate by the difference in moisture content quantified by the method 44-15.02 of the AACC (2017).
Content of starch, glucose, fructose and sucrose
Total starch content was determined in triplicate utilizing the Megazyme kit (Ireland), which is based on the method 76-13.01 of the AACC (2017).
The extraction and quantification of glucose, fructose, and sucrose were carried out following the enzyme-linked assay described by Castañeda-Saucedo et al. (2012). Soluble sugars were extracted from 1 g of tuber or a potato chip sample by six successive incubations of 10 min with ethanol (80 % v/v) at 70 °C. The extracts were evaporated at 50 °C, then re-suspended in distilled water and stored at -20 °C until analysis. Glucose, fructose, and sucrose were quantified in triplicate after the sequential addition of hexokinase (EC 2.7.1.1), phosphoglucose-isomerase (EC 5.3.1.9), and invertase (EC 3.2.1.26) from Sigma-Aldrich® (USA). Absorbances were measured in a Multiskan Ascent microplate reader (Lab Systems®, Canada) at 340 nm against standard curves of glucose, fructose, and sucrose from (Sigma-Aldrich®).
Content of total phenols
The ultrasound assisted extraction method (Joaquín-Cruz et al., 2015) was employed. Ten grams of fresh whole potato tuber was put into a 125 mL Erlenmeyer flask and 30 mL of 80 % methanol were added. The sample was homogenized and sonicated (Branson 2510, Marshall Scientific®, USA) for 30 min. This was then agitated for 90 min in a horizontal shaker at room temperature (25 °C) and under conditions of darkness. The sample was centrifuged at 2 200 g for 15 min, then the supernatant was recovered, and the residue was re-extracted twice with 15 mL of a mixture of methanol:acetic acid:water (10:1:9 v/v/v). All of the supernatants were pooled, filtered and concentrated in a rotavapor (Rotavapor R-215, Buchi®, Switzerland) at 42 °C. Extracts were re-suspended in 5 mL of methanol 80 % (v/v) and stored in dark vials at 4 °C until analysis.
Quantification was performed in triplicate by the Folin-Ciocalteu (Singleton, Orthofer, and Lamuela-Raventós, 1999). An aliquot (100 µL) of the methanolic extract was mixed with 125 µL of Folin-Ciocalteu reagent and allowed to react for 6 min; then the reaction was neutralized by adding 1 250 µL of 19 % Na2CO3 solution and brought to 3 mL with distilled water. The sample was shaken and stored in darkness for 90 min. Absorbance was measured at 760 nm. Total soluble phenolics content was expressed as mg of chlorogenic acid·100 g-1 sample.
Elaboration of potato chips
Potato chips were made as reported by Vázquez-Carrillo et al. (2013). Each tuber was sliced into 1.2 mm thick slices, which were fried for 3 min in vegetable oil at 180 °C in a domestic fryer (F100652, T-FAL®, China). Slices were placed on paper towels to absorb the excess oil.
Yield and physical properties of potato chips
Chip yield was calculated as a percentage by dividing the total weight of the potato chips obtained by the total weight of the tuber processed (Vázquez-Carrillo et al., 2013). Fracturability was evaluated using a texturometer (CT3 25k, Brookfield®, USA) with a spherical probe 1.27 cm in diameter and 3.5 cm in length, whose displacement was at 1 mm·s-1 up to 3 mm distance. A potato chip slice was placed on a base with a central hole 2.5 cm in diameter. Ten potato chip slices were evaluated for each tuber (Vázquez-Carrillo et al., 2013). Chip color was assessed in triplicate with a Hunter Lab colorimeter as reported by Hasbún, Esquivel, Brenes, and Alfaro (2009).
Experimental design and statistical analysis
The experimental design was complete random blocks with four replicates. The treatment design was an asymmetric factorial 4x2x2: the first factor was the genotype (Fianna, Nau, 5-10, and 99-39), the second factor was the location (Metepec and Raíces), and the third factor was the year (2012 and 2013). The experimental unit consisted of 10 tubers. Results were analyzed by analysis of variance, and when significant differences were found a means comparison was conducted by Tukey’s test (P ≤ 0.05) using Statistical Analysis System (SAS Institute Inc., 2002).
Results and discussion
Physicochemical properties of potato tubers
Analysis of variance of the physicochemical properties of tubers showed significant effects (P ≤ 0.05) between genotypes (G), years (Y), and locations (E), except for chroma, in which no significant differences were found between locations (Table 2). All second-order and G x Y x E interactions were significant (Table 2).
Table 2 Analysis of variance of physicochemical parameters of potato tubers.
| SV1 | DF | IBI | Color | Total phenolics | SG | DM | Starch | RS | TS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | Hue | Chroma | |||||||||
| Genotype (G) | 3 | 1.69** | 7.70** | 221.41** | 270.67** | 8184.69** | 0.01** | 11.41** | 17.24** | 0.36** | 0.32** |
| Year (Y) | 1 | 0.15** | 21.50** | 565.82** | 0.25* | 81810.12** | 0.01** | 8.51** | 4.20** | 0.56** | 0.52** |
| G x Y | 3 | 0.02** | 12.90** | 160.88** | 3.01** | 14504.00** | 0.01** | 3.84** | 0.94** | 0.13** | 0.23** |
| Location (E) | 1 | 20.80** | 34.71** | 673.81** | 0.01 | 206146.20** | 0.01** | 58.05** | 43.24** | 1.11** | 2.13** |
| G x E | 3 | 1.69** | 6.23** | 197.96** | 7.55** | 11158.72** | 0.01** | 10.96** | 16.73** | 0.05** | 0.11** |
| Y x E | 1 | 0.15** | 0.83** | 415.30** | 34.84** | 232.20* | 0.01** | 14.18** | 2.31** | 0.75** | 1.31** |
| G x Y x E | 3 | 0.02** | 4.42** | 145.78** | 1.90** | 1208.74** | 0.01** | 2.95** | 3.35** | 0.26** | 0.39** |
1SV = source of variation; DF = degrees of freedom; IBI = internal browning index; L* = luminosity; SG = specific gravity; DM = dry matter; RS = reducing sugars (glucose + fructose); TS = total sugars (RS + sucrose). * = P ≤ 0.05; ** = P ≤ 0.01.
These results indicated that there was an important effect of genetic variability among the genotypes, the climatic conditions in each year, and the altitude of each location, which influenced the prevalence of the ZC symptoms and the accumulation of carbohydrates.
In general, higher internal browning index (IBI) values were observed in 2013 (Table 3), indicating the presence of ZC disease. During this year, there was lower precipitation than in 2012 which could have influenced the ZC incidence. According to Rubio-Covarrubias et al. (2013), in the absence of rain, the population of the insect vector (Bactericera cockerelli) increases, resulting in a greater number of infected plants with tubers exhibiting greater internal browning.
Table 3 Mean comparisons of quality parameters of potato tubers grown in central Mexico.
| Factor | IBI1 | Flesh color | Total phenolics (mg·100 g-1 fresh tuber) | SG | DM | Starch | RS | TS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | Hue | Chroma | (g·100 g-1 fresh tuber) | ||||||||
| Year | 2012 | 0.74 bz | 78.02 b | 88.91 a | 28.55 a | 248.59 b | 1.084 b | 20.35 a | 16.14 a | 0.221 b | 0.449 b |
| 2013 | 0.87 a | 79.66 a | 80.50 b | 28.72 b | 349.72 a | 1.074 a | 19.32 b | 15.42 b | 0.485 a | 0.704 a | |
| Genotype | Fianna | 1.35 a | 78.22 b | 76.94 b | 26.98 b | 266.99 d | 1.085 a | 21.04 a | 16.96 a | 0.282 b | 0.556 b |
| Nau | 0.42 c | 80.31 a | 88.10 a | 26.46 c | 288.81 c | 1.075 b | 19.02 b | 14.32 b | 0.235 c | 0.380 d | |
| 5-10 | 0.42 c | 78.42 b | 87.80 a | 37.13 a | 297.79 b | 1.084 a | 20.66 a | 17.12 a | 0.226 c | 0.517 c | |
| 99-39 | 1.02 b | 78.42 b | 85.99 a | 23.97 d | 343.04 a | 1.072 b | 18.61 b | 14.71 b | 0.671 a | 0.852 a | |
| Location | Raíces | 0.00 b | 79.88 a | 89.29 a | 28.61 b | 218.89 b | 1.083 a | 21.18 a | 16.94 a | 0.167 b | 0.318 b |
| Metepec | 1.61 a | 77.80 b | 80.12 b | 28.66 a | 379.42 a | 1.075 b | 18.49 b | 14.62 b | 0.539 a | 0.835 a | |
1IBI = internal browning index; L* = luminosity; SG = specific gravity; DM = dry matter content; RS = reducing sugars (glucose +fructose); TS = total sugars (RS + sucrose). zMeans with the same letters within each column and between the levels of each factor do not differ statistically (Tukey, P ≤ 0.05).
In Raíces, no tubers had internal browning as expected (Table 3), and these results may be attributed to the altitude and temperatures. Rubio-Covarrubias et al. (2011) reported that the population of the insect vector and ZC disease decrease as the altitude increases; therefore, at an altitude higher than 3 200 m, no significant problems related to ZC have been reported because the low temperatures did not allow the development of the insect vector or reduce its population.
Among genotypes, commercial variety Fianna had the highest values of IBI compared with the clones (Table 3), while clones Nau and 5-10 showed some resistance to internal browning (Table 3). Resistance to ZC disease of clones Nau and 5-10, as well as the susceptibility of Fianna and 99-39 to internal browning has been reported by Rubio-Covarrubias et al. (2013).
Phenolics are important compounds in potatoes because they are associated with the color of the fresh and processed tubers. When tubers are cut, these compounds oxidize and convert into melanins, which enhances the enzymatic browning (Álvarez-Torres & Canet-Parreño, 2009). Total phenolics ranged from 218.89-379.46 mg·100 g-1 fresh tuber (Table 3), similar to values reported by Bradshaw and Ramsay (2009).
Tubers harvested in 2013, and in Metepec, had the highest phenolics content (Table 3), the same trend as shown in the IBI. In fact, the correlation between these variables was positive (r = 0.57, P ≤ 0.01), and this effect is because both factors are symptoms of ZC disease. Wallis et al. (2014) mention that the accumulation of phenols is a defense mechanism against ZC disease. Among genotypes, the highest content of phenolics was found in clone 99-39, which also had a higher IBI, meaning that this genotype is not resistant to ZC disease and that it expresses a higher accumulation of these compounds when infected. Clone 5-10, considered as resistant to ZC disease, had a higher total phenolics content; however, this effect could be due to the presence of anthocyanins, in that its tubers have a red skin (Table 1).
The combination of luminosity (L*), hue, and chroma defined the flesh color. Tubers harvested in 2012, and in Raíces, had clear yellowish flesh, while tubers harvested in 2013, and in Metepec, had flesh with intense yellow-to-orange color. Between genotypes, clones had a yellowish and more whitish flesh than Fianna, and clone 5-10 had the best color (clear yellowish). Flesh color depended mainly on two factors: the IBI and the enzymatic browning produced by oxidation of phenolics, a relation demonstrated by the negative correlations between L* and IBI (r = -0.50, P ≤ 0.01), hue and IBI (r = -0.66, P ≤ 0.01), and between L* and total phenolics (r = -0.43, P ≤ 0.05).
Clones Nau and 5-10 had clear yellowish flesh independent of location or the year during which they were grown. In contrast, the Fianna variety exhibited tones ranging from clear yellowish to intense red. Clone Nau present the same behavior in both flesh color and total phenolics, reaffirming that both variables are principally attributable to the genetic component that provides ZC resistance.
SG is determined by the DM content, which is approximately 80 % starch (Stark & Love, 2003). This relationship was demonstrated by the positive correlations found between SG and DM (r = 0.66, P ≤ 0.01), SG and starch (r = 0.63, P ≤ 0.01), and between DM and starch (r = 0.93, P ≤ 0.01), and these correlations were similar to those reported by Vázquez-Carrillo et al. (2013). The SG, DM, and starch content were higher in tubers grown in 2012, and in Raíces, as well as in the genotypes: Fianna and clone 5-10 (Table 3). The main factors influencing these results were temperature, incidence of ZC, and the maturity of the genotypes.
According to Álvarez-Torres and Canet-Parreño (2009), at lower temperatures, such as those presented in Raíces in both years, the respiration rate is lower than the photosynthesis rate, which increases the accumulation of carbohydrates in the tubers; thus, the SG increases. Additionally, in Raíces, the life cycle was one month longer than in Metepec due to lower temperatures; therefore, plants carried out photosynthesis for a longer time and accumulated a higher starch content. On the other hand, the null ZC incidence of Raíces contributed to the accumulation of starch because the foliage was not damaged.
The genetic influence also played an important role in these results because it was observed that clone 5-10 did not reveal a differentiated response by the effect of the interaction of the remaining factors, that is, accumulation of DM and the SG were similar in all of the assessed environments. The same behavior was observed in the starch content of the clone Nau, although the other DM components, such as protein, lipids, or minerals, could have been affected by the environment; therefore, these changes modified its SG.
Tubers used in the production of chips and French fries must have a DM content higher than 20 %, a starch content higher than 13 %, and SG higher than 1.08 (Stark & Love, 2003); thus, only the Fianna variety and clone 5-10 grown in Raíces were suitable for processing.
Total sugars (TS) content ranged from 0.318 to 0.852 % and reducing sugars (RS) from 0.167 to 0.671 % (Table 3); similar values were reported by Bradshaw and Ramsay (2009) and Hasbún et al. (2009). Tubers grown in Metepec, and in 2013, had the highest content of TS and RS (Table 3). These results are explained by the incidence of ZC. Wallis et al. (2014) reported that tubers infected by ZC disease accumulated higher amounts of RS than healthy tubers as a consequence of the metabolic disorders caused by the causal agent.
Differences among genotypes could also be defined by their genetic code, which determines their metabolism; therefore, clones 5-10 and Nau had lower concentrations of RS and TS than Fianna and clone 99-39 (Table 3). These results could be related with their maturity; clones 5-10 and Nau have early-medium maturity which could influence positively in a lower sugars accumulation. Only tubers from Fianna and clone 5-10 grown in Raíces, in both years (data not shown), had an acceptable quality for chip processing, since tubers for this purpose must have a RS and a sucrose content lower than 0.035 and 0.15 % of fresh tuber, because higher contents give rise to some problems of browning and acrylamide production (Álvarez-Torres & Canet-Parreño, 2009; Stark & Love, 2003).
Physicochemical properties of potato chips
Analysis of variance demonstrated highly significant effects (P ≤ 0.01) in all variables due to the effect of each factor and the interactions among them (Table 4).
Table 4 Analysis of variance of physicochemical parameters of potato chips.
| SV1 | DF | Chip yield | Chip color | Fracturability | Total phenolics | RS | TS | ||
|---|---|---|---|---|---|---|---|---|---|
| L* | Hue | Chroma | |||||||
| Genotype (G) | 3 | 27.04** | 501.63** | 271.65** | 71.47** | 1.29** | 45168.47** | 5.34** | 7.13** |
| Year (Y) | 1 | 10.01** | 380.33** | 736.13** | 98.21** | 2.53** | 21835.27** | 3.24** | 15.05** |
| G x Y | 3 | 9.85** | 160.45** | 44.54** | 18.81** | 0.58** | 15654.02** | 3.51** | 7.74** |
| Location (E) | 1 | 1.95** | 199.20** | 129.12** | 6.62** | 2.42** | 148771.49** | 10.01** | 22.59** |
| G x E | 3 | 7.91** | 57.91** | 38.68** | 7.72** | 0.49** | 36700.84** | 2.87** | 4.47** |
| Y x E | 1 | 1.09** | 10.15** | 4.90** | 30.50** | 1.36** | 24337.69** | 4.67** | 12.63** |
| G x Y x E | 3 | 2.56** | 28.59** | 10.46** | 7.44** | 0.52** | 32766.57** | 4.44** | 8.02** |
1SV = source of variation; DF = degrees of freedom; L* = luminosity; RS = reducing sugars (glucose +fructose); TS = total sugars (RS + sucrose). * = P ≤ 0.05; ** = P ≤ 0.01.
The yield and texture of potato chips depend greatly on tuber density (Pedreschi, 2009). This relationship was confirmed by the positive correlations found between chip yield and SG (r = 0.37, P ≤ 0.05), chip yield and DM (r = 0.60, P ≤ 0.001), chip yield and starch (r = 0.67, P ≤ 0.001), and DM and fracturability (r = 0.37, P ≤ 0.05). Tubers grown in 2012, and in Raíces, as well as tubers with SG > 1.080 and higher DM and starch content gave higher chip yield (Table 5).
Table 5 Mean comparisons for the physicochemical characteristics of potato chips from four genotypes grown in Central Mexico.
| Factor | Chip yield (g·100 g-1 fresh tuber) | Chip color | Fracturability (N) | Phenolics (mg·100 g dry mater-1) | RS1 | TS | |||
|---|---|---|---|---|---|---|---|---|---|
| L* | Hue | Chroma | (g·100 g dry mater-1) | ||||||
| Year | 2012 | 26.1 az | 60.27 a | 79.67 a | 33.42 b | 3.6 a | 207.18 a | 0.448 b | 0.938 b |
| 2013 | 25.0 b | 53.38 b | 70.07 b | 36.42 a | 3.0 b | 154.94 b | 1.084 a | 2.310 a | |
| Genotype | Fianna | 26.7 b | 60.00 b | 76.39 b | 33.26 b | 3.4 b | 211.49 a | 0.424 b | 1.141 c |
| Nau | 24.8 c | 59.00 b | 76.21 b | 34.01 b | 3.7 a | 163.76 c | 0.236 c | 0.862 d | |
| 5-10 | 27.3 a | 63.09 a | 80.28 a | 39.63 a | 2.8 c | 86.10 d | 0.418 b | 1.510 b | |
| 99-39 | 23.3 d | 45.24 c | 66.60 c | 33.78 b | 3.4 b | 262.89 a | 1.984 a | 2.984 a | |
| Location | Raíces | 25.8 a | 59.32 a | 76.88 a | 35.62 a | 3.6 a | 112.87 b | 0.206 b | 0.784 b |
| Metepec | 25.3 b | 54.33 b | 72.86 b | 34.71 b | 3.0 b | 249.24 a | 1.325 a | 2.464 a | |
1RS = reducing sugars (glucose + fructose); TS = total sugars (RS + sucrose); L* = luminosity. zMeans with the same letters within each column and between the levels of each factor do not differ statistically (Tukey, P ≤ 0.05).
The texture of potato chips is a very important quality attribute and this variable in terms of fracturability ranged from 2.8-3.7 N (Table 5), similar values to those reported by Vázquez-Carrillo et al. (2013). Since the correlation coefficient between DM and fracturability was low, it could be inferred that not only DM components such as starch influenced chip texture, but also the composition and structure of the cell wall, especially the presence of pectic substances; however, more research is needed on this aspect.
Potato chips with the best yellowish color (higher values of L*, hue, and chroma) were produced from tubers grown in 2012, in Raíces, and from clone 5-10, as expected (Table 5). The inverse trend was observed in potato chips from clone 99-39, which were dark brown in color. Several compounds influence potato chip color, including RS, sucrose, ascorbic acid, chlorogenic acid, glutamine, lysine, glycine, and arginine (Rodriguez-Saona & Wrolstad, 1997). Luminosity and hue were well correlated with the RS content of fresh tubers (r = -0.67 and r = -0.68, respectively, P ≤ 0.001), as well as with total phenolics of fresh tuber (r = -0.65, P ≤ 0.001 and r = -0.66, respectively, P ≤ 0.01), meaning that the higher content of RS and total phenolics in fresh tuber enhanced the non-enzymatic browning of potato chips. For this reason, clone 99-39 had dark-brown potato chips, while clone 5-10 had clear yellowish potato chips.
Total phenolics in potato chips ranged from 14.5-532.5 mg·100 g-1 (Table 5). The loss of these compounds ranged from 60.4 to 98.4 % with respect to fresh tubers. Higher losses were recorded in tubers from genotypes with lower contents. Kalkan and Yücecan (2013) indicated that losses of phenolics after frying ranged from 20 to 76 % and the main compound lost is chlorogenic acid.
The RS and TS contents decreased with respect to tuber content, and part of these sugars were utilized in the non-enzymatic browning reactions giving the color of potato chips. Our values (Table 5) were similar to those observed by Roe, Church, Pinchen, and Finglas (2013), who reported values ranging from 0.3 to 1.5 g·100 g-1 of TS in potato chips obtained by means of several frying methods.
Conclusions
Differences between years, and among locations and genotypes, as well as the interaction among all of the factors influenced the quality of tubers and potato chips. Precipitation, altitude, temperature, and the resistance of the genotypes influenced the zebra chip disease symptoms. Thus, the highest precipitation, lowest temperatures, and the use of resistant genotypes led to the production of tubers with the lowest internal browning and highest starch and dry matter content, as well as the lowest reducing sugars and phenolics content. These conditions allowed obtaining suitable tubers for chip processing, giving chips with acceptable color and texture. Clone 5-10 grown in Raíces in both years had the best physicochemical characteristics for fresh consumption and chip processing.











 texto en
texto en