Introduction
Loxaulus is a Nearctic genus of gall wasps that grows on Quercus (Melika & Abrahamson, 2000); 15 species have been reported from the USA (Melika et al., 2021), two from Panama (Medianero et al., 2011) and two from Mexico (Pujade-Villar et al., 2014).
Biology studies are indispensable to understand the relationships between phytophagous, hosts and associated species. In 13 species of Loxaulus only asexual generation is reported, of which 10 induce their galls on twigs (Loxaulus atrior [Kinsey] Dailey 1977, Loxaulus boharti Dailey and Sprenger 1983, Loxaulus brunneus [Ashmead 1896], Loxaulus championiMedianero y Nieves-Aldrey 2011, Loxaulus maculipennis [Ashmead 1896], Loxaulus tenuis [Weld 1952], Loxaulus vaccini [Ashmead] Weld 1951, Loxaulus panamensis Medianero & Nieves-Aldrey 2011, L. laetaPujade-Villar 2014, Loxaulus virginianaeMelika & Buss 2021), one on leaves (Loxaulus beutenmuelleri Weld 1957), one on roots (Loxaulus pattersoni [Kinsey 1922]) and one on belowground stems (Loxaulus illinoisensis [Weld 1921]) (Medianero et al., 2011; Melika & Abrahamson, 2000; Melika et al., 2021; Pujade-Villar et al., 2014). Sexual generation has been reported only in Loxaulus quercusmammula (Bassett 1881) and Loxaulus hyalinus Pujade-Villar and Melika 2014 (in this study) in galls induced in twigs. The females of Loxaulus ferrugineus (Gillette 1891), Loxaulus huberi Melika and Abrahamson 2000, and Loxaulus masneri Melika and Abrahamson 2000 are known, but the galls and to which generation they correspond are unknown (Melika & Abrahamson, 2000). Both asexual and sexual generation are known only from Loxaulus trizonalis Weld 1926, the first inducing galls on acorns and the latter on twigs of Quercus chrysolepis Liebm. 1854 (Melika & Abrahamson, 2000).
In 2014 Loxaulus laetaPujade-Villar 2014 was reported on Quercus laeta Liebm. 1854 (section Quercus) in Santa Fe, Cuajimalpa, Mexico City and L. hyalinus on Quercus dysophylla Benth. 1840 (section Lobatae) in Huasca de Ocampo, Hidalgo (Pujade-Villar et al., 2014). Recently, the identification of the host for L. hyalinus was corrected to Q. laeta by Cuesta-Porta et al. (2022), meaning that the two species known from Mexico attack Q. laeta. It has been found that the L. hyalinus galls cause alterations in the growth of infested branches, as well as in the structure and appearance of the host crown, so this species is considered a pest in green areas of residential neighborhoods in Mexico City.
The galls of oak trees produced by phytophagous wasps are exploited by various organisms, including inquilines and parasitoids that compete for the host or attack it (Todorov et al., 2019). In galls produced by cynipids, chalcidoid parasitoids are, in general, the most important natural enemies, while species of the tribe Synergini (Cynipidae), lethal or non-lethal (facultative), are the main group of inquilines (Sanver & Hawkins, 2000).
The aim of the present study was to describe the development stages of the sexual generation of L. hyalinus, its galls, type of host damage and to identify the associated inquiline species and parasitoid genera.
Materials and Methods
The study area was located in the Barranca ‘Los Helechos’ (Figure 1a), Santa Fe, San Mateo Tlaltenango, Cuajimalpa, Mexico City (between 19° 21’ 16.7” - 19° 20’ 52.1” N and 99° 16’ 38.7” - 99° 15’ 40.1” W), at 2 547-2 623 m elevation. Quercus laeta is found in a natural forest area, at the edge of roads, around buildings and several common areas (Figure 1b).
Another study site was the municipality of Huasca de Ocampo, Hidalgo (20” 12’ 12.34” - 20° 12’ 14.51” N and 98° 34’ 14.64” - 98” 34’ 06.76” W (Figure 1c). On 8 and 29 May 2021, L. hyalinus twig galls and host samples (green marks, Figure 1d) were collected in the vicinity of the site reported by Pujade-Villar et al. (2014) (yellow mark, Figure 1d). Galls were placed in individual containers and host samples were placed in a plant press, dried and identified. The adults that emerged from galls were placed in 2 mL Axygen® microtubes with 96 % ethyl alcohol.
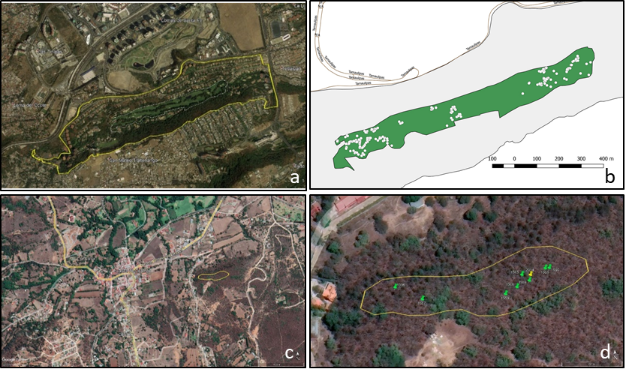
Figure 1 Study sites for collecting Loxaulus hyalinus in Quercus laeta oak groves: a) Santa Fe, Cuajimalpa, Ciudad de México; b) trees under observation marked with white dots within the green polygon; c) Huasca de Ocampo, Hidalgo; d) polygon examined, hosts studied (green mark) and site reference point (yellow mark).
The development of immatures and the timing of adult emergence were studied on galls collected from October 2019 to June 2021 in Santa Fe. Field surveys were conducted at least once a month and twigs were collected to look for eggs or young galls. Collections were carried out at least once a week when pupae began to be observed in March of each year and until adult emergence was completed in the laboratory.
Loxaulus hyalinus and its galls were observed in the laboratory of Insect Physiology of Fitosanidad-Entomología y Acarología, Colegio de Postgraduados, Campus Montecillo, Texcoco, Estado de México, México. For each date collection, three galls were selected from each of five trees. Bark and parenchyma were removed with a razor until larval chambers were found; content of each larval chamber was extracted and the type of organism and its stage of development were recorded. Larvae and pupae were stored in ethyl alcohol (96 %). A group of larvae of all sizes was associated with the dates of collection and their morphological characteristics were described, just as in the pupal stage, where changes during their development, body length and their presence in relation to the dates of collection were also recorded. Eggs, larvae, pupae and larval chambers were measured using Adobe Photoshop® CS3.
The beginning of the adult emergence period was recorded from the detection of the first pupae in the dissected galls until the first week of June 2019 to 2021. From each collection date, at least 20 galls were individually placed in 1.0 L clear plastic containers, which were considered emergence chambers, and an identification number was assigned to them. They were kept under room temperature and natural light conditions. As soon as the first adults of L. hyalinus were found in the dissected galls, the chambers were checked daily to collect and transfer them with an aspirator to a 1.0 L transparent container with ventilation cover, per day, to observe their behavior. Detected parasitoid and inquiline adults were captured and preserved in microtubes with ethyl alcohol (96 %), one microtube/chamber/day. In 2020 and 2021, a plastic bottle cap with water and honey drops as food, and Q. laeta leaves for shelter were placed inside the containers, which were inspected until no adult emergence was recorded.
Adult longevity was determined on 49 individuals emerged between 9 and 15 May 2020 under laboratory conditions. The containers were checked daily at 10:00 and 15:00 h, to record feeding and sexual behavior. The containers remained closed until all the adults were dead, and then they were placed in 96 % ethyl alcohol. The number of L. hyalinus, females and males, parasitoids, and adult inquiline cynipids captured per chamber/collection date of 2019, 2020, and 2021 was recorded. Females of L. hyalinus were identified based on the description of Pujade-Villar et al. (2014) and males based on data from Cuesta-Porta et al. (2022).
The number of developed eggs was counted in 14 females with dilated abdomen following the procedure of Hood and Ott (2011). The abdomen was detached from the body, the integument of the second and third segments was removed and the abdomen was placed on a reaction plate with a 1:1:13 solution of acetic acid, glycerol and water, respectively, for a period of at least 2 h to loosen the follicular tissue of the ovary. Eggs were separated from the ovarioles and counted using UV light (395 nm) under a microscope. From each female, 10 eggs were taken at random and placed on a slide with a 70 % glycerol solution and photographed. Their body length, body width and peduncle length were measured according to Vårdal et al. (2003).
The length of the gall, its minimum and maximum diameter, diameters of the twig before and at the end of the gall, and distance from the gall to the terminal bud of the twig were measured with an iGaging® OriginCal® caliper (resolution = 0.01 mm) on 70 galls collected in February 2022 and the number of larval chambers was counted. The correlation matrix (P = 0.05) of the measured variables and the multiple linear regression (α = 0.05) of gall length - number of larval chambers were calculated with STATISTICA® software version 8.0 (StatSoft Inc., 2007).
The structure of two twigs collected in February 2022, with and without typical galls of L. hyalinus, was described from 25 µm to 30 µm thick longitudinal and transverse micrometric sections fixed in formaldehyde-alcohol-acetic acid and sectioned with a Leica® SM2000R slide microtome. The sections were stained with Bismarck Brown, which stains cellulose from plant cell walls (Garrido-Fariña et al., 2021) from different tissues, such as phloem (Blaydes, 1939).
The effects of L. hyalinus infestation on twigs of Q. laeta were recorded on trees located in highly disturbed sites with evident infestation between 2018 and 2021. Observations on twig survival, presence of foliage and apical dominance were included. Parasitoids were identified to genus level with the keys of Gibson et al. (1997), and the species of the inquiline cynipids were identified based on information from Lobato-Vila and Pujade-Villar (2017, 2018) and Lobato-Vila et al. (2019, 2020).
Field photographs were taken with a Sony® A7R3 digital camera and a Vario-Tessar® T* FE 24-70 mm F4 ZA OSS lens, as well as in the laboratory with the same camera and 4x, 10x and 20x planar lenses in the Remote (Sony®) program. A scale with divisions at 0.01 mm from a micrometer calibration slide was used as a reference for the measurement. The photographs were processed with Helicon Focus 7 (Heliconsoft®), Capture One® 20 and 22 and Adobe Photoshop® CS3. Statistical tests, correlation matrices, multiple linear regression and calculation of descriptive statistics were performed with STATISTICA® 8 (StatSoft®).
Results and Discussion
In 2019, females and males of L. hyalinus emerged from twig galls of Q. laeta collected in Santa Fe. Previously, Pujade-Villar et al. (2014) reported L. hyalinus from twig galls of Q. dysophylla, collected in Huasca de Ocampo, Hidalgo, but they only described the females and indicated that they belonged to the asexual generation; however, in 2021 they made another collection in Huasca and found branch galls of Q. laeta that produced females and males of L. hyalinus, but not in Q. dysophylla. It is important to mention that the two Quercus species belong to different sections and that Q. dysophylla showed no twig galls in that locality, which suggests that there was an error in the identification of this species as a host. In 2014, the same authors mentioned that, in Santa Fe, L. laeta generated twig galls and that it was the main branch galls species of Q. laeta; in contrast, for all collections carried out in this study, between 2019 and the first quarter of 2022, only females and males of L. hyalinus were collected. According to the authors mentioned above, the morphological difference between the wings of L. hyalinus (completely hyaline) and L. laeta (with a characteristic spot) is easily distinguishable.
Developmental stages of Loxaulus hyalinus
The asexual form occurs between two consecutive sexual generations. In the sampling periods from June to October, twigs were examined for the ovipositions of the asexual form that give rise to the galls of the sexual generation, but they were not found. Therefore, it is still unknown in which host organ the galls of the asexual generation are found, their appearance and when the agamic females oviposit, so that, in this study, only the developmental stages of the sexual form are described.
Larvae
From October to December, longitudinal sections of developing galls showed, in zenith view, that the chambers were filled with nutritive tissue. In this period, the L1 instar is a translucent sphere without external segmentation located in the center.
In the January observations, 57.1 % of the gall-inducer larvae corresponded to L1 with reduced activity and could be found in different places inside the chamber, while the nutrient tissue no longer covered the entire interior and 42.9 % corresponded to L2.
The larvae of L. hyalinus from instar L2 are hymenopteriform with a fusiform body, curved ventrally, with the anterior part slightly wider than the rest, without projections, whitish, smooth and shiny, with little evident segmentation in the first instars and 12 visible segments of similar size in the more developed instars (Figure 2a), similar to other species of Cynipoidea, as described by Nieves-Aldrey et al. (2005). The head is small especially in the first instars, with tiny, single-toothed mandibles with a sharp tip (Figure 2b); Nieves-Aldrey et al. (2005) indicate that the number of teeth in Cynipoidea varies from one to five between the left and right mandible.
Pupa
In April, last instar larvae and pupae of L. hyalinus with different levels of development were found in the galls (Figure 3a). The pupa is exarate and as development progressed it was possible to differentiate the body tagma, legs and antennae (Figure 3b). By mid-April, development was complete. Pigmentation begins in the eyes and continues on the rest of the body (Figure 3c); the wings were the last to form.
Preimagos, adult and egg
Preimagos with developing wing packets could walk steadily when extracted from the chambers. Non-emerged adults when extracted from the chambers or when they emerged naturally began to spread their wings as their first activity.
Loxaulus hyalinus is black with a massive head (1.9 times wider than long in dorsal view in females and 1.7 to 1.8 in males) with malar space 0.2 times the height of the compound eye. Females (Figure 3d) have 13 antennal flagellomeres with placoid sensilla visible from segment F3; while males (Figure 3e) have 15 flagellomeres; F1 0.8 times F2 and slightly curved; posterior lateral ocellus with a transverse impression; mesosoma is 1.4 to 1.5 times longer than wide in lateral view; notauli incomplete, scutellar pits longitudinally carinate, forewings translucent with setae on margin, radial cell 3.0 times longer than wide, and ventral spine of hypopygium short and as long as wide (Cuesta-Porta et al., 2022; Pujade-Villar et al., 2014).
Adult survival was one to two days when they lacked shelter, water and carbohydrates, while those with these resources lived four to six days.
In the field, mating was observed on May 18, 2021, around noon, under clear weather conditions. Adults remained on the foliage and stem of nearby Q. laeta individuals and at ground level; active adults were found mainly on the underside of leaves, but oviposition or attempts at oviposition by females were not recorded. There was no mating in the laboratory, due to low light intensity conditions.
For the 14 females of the sexual generation, the abdomen was almost entirely occupied by the ovarian egg mass (Figure 3f) and contained on average 57.78 ± 5.50 eggs per female. These were observed translucent to whitish under white light (Figure 3g) and fluorescent green under UV light (Figure 3h); the body and peduncle are distinguishable. The body is longer than wide at a ratio of 3.7:1.0, with the rounded distal end gradually tapering toward the peduncle, which is approximately 1.6 times longer than the body (Table 1) and shows a gradual widening toward the distal end (Figures 3g and 3h). The general characteristics of the ovarian egg agree with the description of eggs of other cynipid galler species, according to Vårdal et al. (2003).
Table 1 Dimensions of ovarian eggs of Loxaulus hyalinus (n = 14).
| Variable (mm) | Average | Minimum | Maximum | DE | IC-Lower limit | IC-Upper limit |
|---|---|---|---|---|---|---|
| Body length | 0.2355 | 0.1313 | 0.2849 | 0.0203 | 0.0181 | 0.023 |
| Body width | 0.063 | 0.0318 | 0.0822 | 0.0082 | 0.0074 | 0.0093 |
| Peduncle length | 0.3746 | 0.177 | 0.6377 | 0.0714 | 0.0639 | 0.0809 |
| Total egg length | 0.6101 | 0.401 | 0.8721 | 0.0736 | 0.0659 | 0.0834 |
SD: standard deviation; CI: confidence interval.
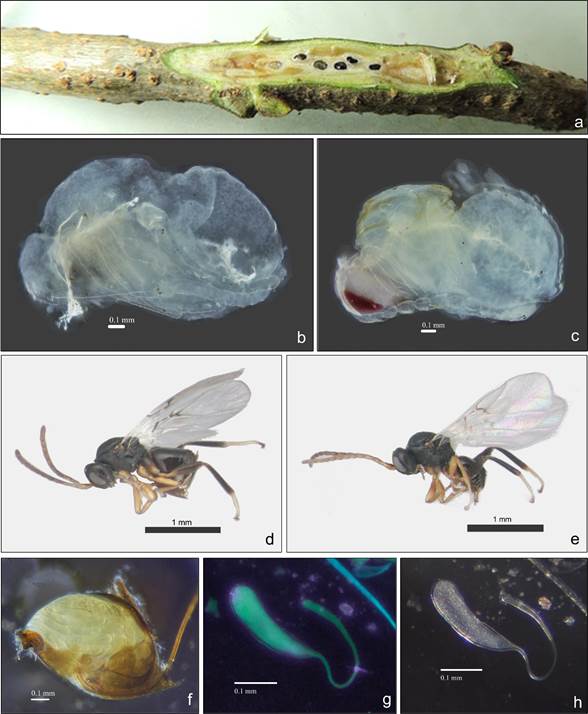
Figure 3 Characteristics of the development stages of Loxaulus hyalinus: a) gall with larvae and pupae; b and c) pupae with different degree of development; d and e) female and male, respectively; f) female oviplene metasome; g and h) ovarian eggs under white light and UV light (395 nm), respectively.
Characteristics and development of twig galls
Because no oviposits were found before September, the timing of when females of the asexual form of L. hyalinus (still unknown) oviposit on twigs to initiate the sexual phase of the cycle and the characteristics of galls in early stages of growth were not determined. In October, galls were observed as elongated, inconspicuous swellings. By January of the following year, the galls developed an elongated shape with slightly rough to rough bark, and a coloration that ranged from brown to greenish-gray, similar to the basal and distal portion of the not infested twig (Figure 4f). In June, after adult emergence, the mature galls turn grayish brown (Figure 4g). According to Stone and Schönrogge (2003) and Hernández-Soto et al. (2015), two regions can be distinguished in the galls; the internal one, composed of the larval chambers, and the external one, by cortical tissue. Loxaulus hyalinus galls show contiguous chambers only interrupted by less developed areas in which there are no chambers, or these are individual and isolated with external, poorly developed gall tissue giving an appearance of adjacent swellings (Figure 4f).
An average gall length of 40.91 mm (minimum = 9.75 mm, maximum = 108 mm) was observed in 70 galls measured in January 2022. The average gall diameter was 4.94 mm (minimum = 4.42, maximum = 5.46 mm) and that of the twig before initiating the gall was 3.42 mm and after ending distally was 2.82 mm. Galls ended on average 16.3 mm from the terminal bud of the twig. Forty galls had green leaves and the rest had brown leaves (10) or no leaves (20). The average number of chambers per gall was 36.4 with an interval from 7 to 104 and significant positive correlation of 0.80 (P < 0.05, n = 70) between length and number of chambers, although there were long galls with a low number of chambers and vice versa. Multiple linear regression provided R2 = 0.6408 which corroborated the relationship.
In L. hyalinus, the internal gall is the larval chamber, whose structure is composed from the inside out by a layer of nutritive tissue and a layer of parenchyma that totally or partially cover the interior, and a layer of sclerenchyma that delimits them and to which vascular bundles join in the basal region. The external gall has a similar structure to the branch without gall (Figure 4b), but its parenchyma is thicker, especially around the larval chambers (Figures 4c, 4d and 4e). The chambers are aligned longitudinally to the axis of the branch, in contiguous groups of two to six, in four to five semi-straight and sometimes helical rows, although there are variations attributable to the number of eggs initially deposited (Figure 4a).
A typical chamber has an oval shape in zenith view, with average values of 2.8 mm long and 1.4 mm wide (n = 30), with the most elongated part parallel to the direction of twig growth (Figure 4f and 4g). Other less frequent forms are semicircular, smaller than oval (length: 1.25 ± 0.17 mm; width: 1.49 ± 0.16 mm; n = 8), and the irregular, in which deformation is observed on some of the sides, but the tissue structure is preserved (Figure 4a). In the basal part of the center of the chamber there is a conical cavity that connects with the heartwood of the twig. The periphery of the chamber is formed by a coriaceous layer of sclerenchyma that inwardly connects with the parenchyma, which, by effect of larval feeding, becomes nutritive tissue, as suggested by Melika (2006). In transverse position, the gall shows drop-shaped chambers (length: 1.91 ± 0.05 mm; width: 1.23 ± 0.19 mm; n = 42), with the narrow part inserted deeply into the xylem (Figure 4c and 4d), which, when there is no nutritive tissue, in zenith view is perceived as a conical cavity prolonged towards the center of the twig (Figure 4h and 4i). The inducer is actively involved in gall development and differentiation (Stone & Schönrogge, 2003). Like other cynipids, galls of L. hyalinus are formed by host tissue whose function and structure are modified (Harris & Pitzschke, 2020), as observed in perpendicular sections of branches with and without galls (Figure 4b and 4c).
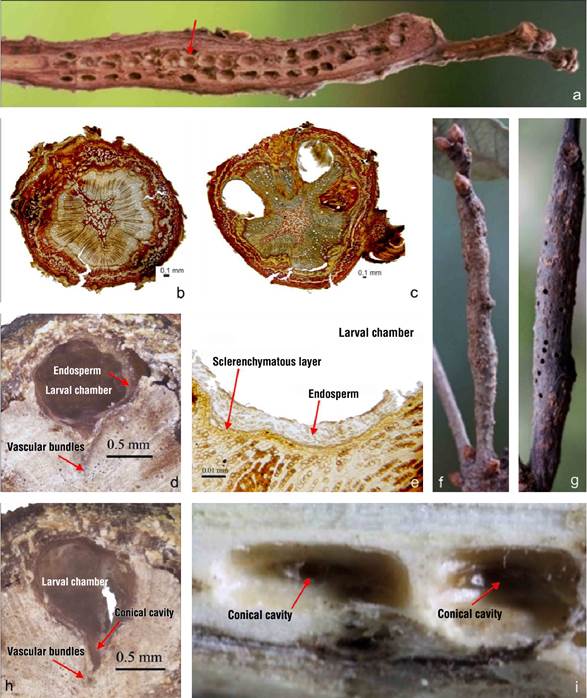
Figure 4 Galls of Loxaulus hyalinus: a) barkless gall with exposed chambers (arrow); b) transversal section of branch without galls; c, d and e) transversal section of a branch with galls and mature larval chambers; f) developing gall; g) mature gall with emergence holes; h) larval chamber in transversal view; i) larval chambers in zenith view. Arrows indicate the conical cavity penetrating the xylem.
Life cycle of sexual generation
Because a biological cycle with only sexual generation is very rare in Cynipini, it is hypothesized that the known generation of L. hyalinus comes from ovipositions of females of asexual origin, which were not found in this study.
In October and November, when small galls were detected, the development stage was L1, which, in longitudinal sections and in zenith view, is observed as a translucent sphere. In the first half of January, the chambers completed their development and the larvae began to develop. The larval stage lasted until mid-April. Pupae were most frequently detected in the first two weeks of April, while preimagos were observed until the last week of April.
In the years where adult emergence was recorded, variations of a few days for its beginning and weeks for its end were found. Emergence of adult females and males began during the last week of April and lasted until the last week of May 2019, 2020 and 2021 and, exceptionally, until the second week of June 2020. The highest male emergence occurred between 1 and 7 May, while that of females was between 26 April and 3 May.
Mating was observed only in the field on 18 May 2021 around noon and under clear sky conditions. At the study site, active adults were found mainly on the underside of leaves, although oviposition was not observed.
Sexual generation of L. hyalinus requires at least eight months and occurs from October to May of the following year. Since we do not know where the asexual phase develops, the total duration of the biological cycle in its two phases remains uncertain.
Consequences of the impact of Loxaulus hyalinus
In the study site, the distribution of Q. laeta is fragmented with the greatest number of individuals located in a residual forest, on roads, in parks and on a golf course. Distribution and damage of L. hyalinus is higher in disturbed sites where oak trees suffer stress of various types, either by construction impacts or by being in soils exposed to erosion and nutrient washing, as well as lack of water. Affected trees have different sizes and compact, deformed crowns and 'zigzagging' branches, a consequence of chronic infestations over several years.
The presence of galls in Q. laeta can cause, in addition to foliage loss (Figure 5a), smaller branch size (Figure 5b) and impairment of water and nutrient conduction, so that only a few twigs can continue with normal development. Twig death during gall development or after adult emergence, and loss of apical dominance with subsequent sprouting of lateral buds at the base of the infested branch, result in a wavy growth pattern and a tortuous crown appearance (Figure 5c and 5d). Tooker et al. (2008) indicate that gall inducers consume resources that hosts could use for growth and reproduction, suppress or manipulate their defenses, alter their physical characteristics, impact their phenotype by changing patterns of photosynthetic rate and biomass accumulation, and stimulate special tissues for protection of the inducer, which can cause profound effects on their hosts. A high presence of L. hyalinus has an important visual effect on sites where oaks are important elements of the landscape, because the archetypal pattern of unattacked Q. laeta is lost.
Inquilines and parasitoids of Loxaulus hyalinus
The inquiline cynipids in the galls of L. hyalinus have at least two emergence periods: one from late January to March and another coinciding with the emergence of the gall inducer. In February 2022, during the larval stage of L. hyalinus, the emergence of Synergus pseudofilicornisLobato-Vila and Pujade-Villar 2018 (previously named Synergus filicornis Cameron, 1883) was observed, and from April to May (L. hyalinus emergence period) of 2020 and 2021, of Synergus grahami Lobato-Vila and Pujade-Villar, 2019. Synergus macrackenaeLobato-Vila & Pujade-Villar, 2021 (Lobato-Vila & Pujade-Villar, 2021) and S. filicornis in branch galls of Loxaulus (Lobato-Vila et al., 2020) have also been reported. Because only the emergence period of L. hyalinus was evaluated in detail, the effect on mortality may be expected to be greater than observed, as these are lethal inquilines.
In a collection on 26 April 2019, 279 adults of different species were obtained from two galls. Emergence was recorded from April 28 to May 12. The individuals corresponded to Eurytoma (135 adults, 48.49 %), L. hyalinus (117 adults, 41.93 %), Synergus (12 adults, 4.30 %), Sycophila (nine adults, 3.22 %), Ormyrus (one adult, 0.36 %), Pteromalidae (three adults, 1.07 %) and Eulophidae (two adults, 0.72 %).
From April 8 to June 18, 2020, 652 adults of different species emerged in 30 galls; the largest number of these emerged between May 10 and 27. The most numerous group was Eurytomidae (337 adults, 51.69 %), followed by Pteromalidae and Eulophidae (147 adults together, 22.55 %), L. hyalinus (90 adults, 13.80 %), Synergini (66 adults, 10.12 %), Ormyridae (10 adults, 1.53 %) and Eupelmidae (two adults, 0.31 %).
Between April 20 and May 17, 2021, 488 adults emerged from 27 galls; of these, the most numerous insect group was Eurytomidae (224 adults, 45.90 %), followed by L. hyalinus (190 adults, 38.93 %), Synergini (15 adults, 3.07 %), Eulophidae (33 adults, 6.76 %), Pteromalidae (12 adults, 2.46 %), Ormyridae (nine adults, 1.84 %), Torymidae (four adults, 0.82 %) and Eupelmidae (one adult, 0.20 %).
The genera of chalcidoid parasitoids in the branch galls of L. hyalinus were: Eurytoma and Sycophila (Eurytomidae), Ormyrus (Ormyridae), Torymus (Torymidae), Eupelmus (Eupelmidae), Pteromalus (Pteromalidae) and Eulophidae. The emergence of Encyrtidae and Platygastridae, which may not be associated with L. hyalinus and its natural enemies, was also recorded. The most diverse parasitoids are Eurytoma and the families Pteromalidae and Eulophidae, which have multiple morphospecies. The remaining genera are less diverse, with at least one morphospecies.
In the years studied, a great diversity of natural enemies could be recognized, whose natural control effect on L. hyalinus could be significant, as suggested by the decrease in the number of galls, although there is no conclusive information on the cause of this reduction.
In 2019 and 2020, high presence of L. hyalinus galls was recorded on the young branches of some individuals of Q. laeta. In 2021, a reduction of branch galls was observed, probably because of natural enemies or other environmental impacts in previous years, although the higher presence of L. hyalinus in that year could indicate that the populations of the inducer, inquilines and parasitoids interact irregularly.
In the dissection of galls collected from January through the second week of April 2020 and 2021, immature stages of the inducer, inquilines, and parasitoids were found. In January 2022, in addition to L1 larvae of L. hyalinus in chambers with characteristics similar to those of the inducer, last instar cynipid larvae were found with mandibles with three teeth (the largest apical), similar to the larvae of S. filicornis described by Tormos et al. (2014).
Conclusions
This research allowed us to know the characteristics of the galls of Loxaulus hyalinus and its role as an inducer of twig galls that affect growth, structure and appearance of Quercus laeta, but without causing its death. There is a great diversity and number of chalcidoid parasitoids and cynipid inquiline associated to L. hyalinus, the most numerous are Eurytoma and Synergus, although the magnitude of the effect of these organisms on the population of the galler is unknown. Even though asexual generation of L. hyalinus was not found, it is possible that it is present.











 texto en
texto en 





