Highlights:
Rhynchophorus palmarum was captured on the genotypes ‘Alto Pacífico ecotipo 2’ and ‘Enano-Verde de Brasil’.
In ‘Enano-Verde de Brasil’ 3.5 times more insects were captured (4 799) than in ‘Alto Pacífico’.
Trash can traps and Colegio Superior de Agricultura Tropical had better cost-benefit.
Correlations of climate variables with capture were weak (less than 50 %).
Introduction
The black palm weevil, Rhynchophorus palmarum L. (Coleoptera: Curculionidae), is the most important pest in coconut (Cocos nucifera L.), date palm (Phoenix dactylifera L.) and oil palm (Elaesis guineensis Jacq.) plantations in the Americas (Rodríguez-Currea, Marulanda-López, & Amaya, 2017; Silva-Dionisio et al., 2020). The insect's distribution ranges from southeastern California and Texas to Argentina, Paraguay, Uruguay, and Bolivia (Aldana de la Torre, Aldana de la Torre, & Moya, 2010; Oehlschlager, Chinchilla, Castillo, & González, 2002).
In México, Landero-Torres et al. (2015) and Murguía-González et al. (2017) reported the incidence of R. palmarum in ornamental palm crops such as Whashingtonia robusta Wendl, Livistona australis (R. Br.) Mart. and Dypsis lutescens (H. Wendl.) Beentje & J. Dransf. Adult females of R. palmarum deposit their eggs in the crown of the palms and the larvae produce galleries in the apical meristem. The economic threshold is 20 larvae per palm, which is enough to cause a lethal infestation. Moreover, the adults are the vector of the nematode Bursaphelenchus cocophilus (Cobb.), causal agent of the 'red ring' disease in C. nucifera plantations (Cysne, Cruz, Cunha, & Rocha, 2013; Sumano, Sánchez, Romero, & Sol, 2012).
In Pacific-central Mexico (Colima, Michoacán and Jalisco), the association of R. palmarum and B. cocophilus is the main phytosanitary problem for coconut production. This complex causes losses of up to 80 % in young plantations (less than four years old), including hybrid genotypes and ecotypes (Sánchez-Ríos, Gutiérrez-López, Aragón-Robles, & Córdova-Gámez, 2017). Studies have been carried out to find efficient control methods for this complex; however, the application of chemical insecticides (carbaryl, carbofuran, fipronil, imidacloprid, and phenylpyrazole) continues to be the main strategy by producers, despite the environmental drawbacks associated with the use of these chemically synthesized molecules. As alternatives, some producers employ mechanical and ethological control using traps with aggregation pheromone (2 (E) -6-metil-2-hepten-4-ol) (Rhynchophorol, Pherocon®) and food baits (Abdelazim, Aldosari, Mumtaz, Vidyasagar, & Shukla, 2017; Hoddle & Hoddle, 2015; Murguía-González et al., 2017). The application of entomopathogenic fungi as biological control (Alencar-Lima et al., 2020; Carreño-Correa, Salazar-Mercado, & Espinel-Rodríguez, 2013; León-Martínez, Campos-Pinzón, & Arguelles-Cárdenas, 2019) and cultural control by knocking down and destroying infected palms (Carreño-Correa et al., 2013; Quintero, 2010) are occasionally applied.
Mechanical control of R. palmarum using pheromone traps is the most appropriate strategy for managing this pest, due to its ethology and biological cycle. Therefore, it is important to evaluate trap designs with the purpose of massive trapping of R. palmarum adults and thus reduce damage in coconut cropping systems (monoculture and associated crops). Therefore, finding an effective and low-cost trap is a major objective of the study, since many coconut growers have small properties and lack information that would motivate them to use a particular trap. On the other hand, the role that environmental conditions (solar radiation, precipitation, temperature, wind speed, among others) play in the efficiency of mass trapping of R. palmarum is unknown (Cysne et al., 2013; Ferreira, Leal, Sarro, Araujo, & Moura, 2003; Landero-Torres et al., 2015).
The objectives of this research were to determine the efficiency of trapping R. palmarum and its cost-benefit in two coconut plantations ('Alto Pacífico ecotipo 2' and ‘Enano-Verde de Brasil'), as well as to correlate mass trapping with environmental factors.
Materials and Methods
Study area
The study was carried out in two coconut orchards. The first corresponded to the genotype 'Alto Pacífico ecotipo 2' with a size of 56 ha including plants from five (60 %) to 30 years (40 %) of age. This variety initiates flowering between 5 and 7 years, fruits at 2.5 m, has heights of 25 to 30 m and produces up to 123 fruits∙palm-1∙yr-1 (Flores López et al., 2014). The orchard was located at 10 m elevation in the municipality of Armería, Colima, Mexico (18° 54' 59.2" N; 103° 59' 39.3" W), in a warm sub-humid climate with an average temperature of 26.4 °C, maximum and minimum of 30 °C and 18 °C, respectively, relative humidity of 86 % and precipitation of 790 mm∙yr-1. The second orchard corresponded to the genotype ’Enano-Verde de Brasil' with an area of 26 ha, located in Caleras, Tecomán, Colima, Mexico (18° 59' 29.08" N; 103° 53' 21.16" W). This plantation included two-year-old plants, an age when flowering begins, fruit at 0.8 m, have heights of 10 to 12 m, and produce up to 200 fruits∙palm-1∙yr-1 (Fundación para el Desarrollo Tecnológico Agropecuario y Forestal de Nicaragua [FUNICA], 2000). The orchard was located at 74 m in a warm sub-humid climate with an average temperature of 26 °C, maximum temperature of 30 °C and minimum of 18 °C, annual thermal oscillation of 13.5 °C with relative humidity of 88 % and precipitation of 790 mm∙yr-1.
Rhynchophorus palmarum trapping
In the orchard with ‘Alto Pacífico ecotipo 2’, the efficiency of the traps for mass trapping of R. palmarum adults was evaluated with five types of traps with six replicates each (Figure 1). The designs were established based on scientific literature: bucket type (Murguía-González et al., 2017), trash can (Ramos et al., 2017), gallon type (Moya-Murillo, Aldana-De la Torre, & Bustillo-Pardey, 2015; Rodríguez-Currea et al., 2017), CSAT trap (Colegio Superior de Agricultura Tropical, Tabasco, Mexico) and bottle type (Murguía-González et al., 2017). The traps were placed on the perimeter of the orchard 100 m from each other and were randomly distributed. Pieces of sugarcane (200 g) and molasses (100 mL) were used for each trap as food bait that was replaced every 15 days and a capsule of Rhynchophorol (Pherocon®) as aggregation pheromone that was replaced every three months in the sampling year. Insects were collected every week starting in August 2018 and ending the same month in 2019. The total insect catch was recorded, as well as the number of females and males.

Figure 1 Trap positions (BT = bucket type, TCT = trash can trap type, GT = gallon type, CSAT = Colegio Superior de Agricultura Tropical trap, BT = bottle type) at the experimental area of the coconut orchard with the genotype 'Alto Pacífico ecotipo 2' in Armería, Colima, Mexico.
The population fluctuation of R. palmarum trapping and its correlation with climate variables was analyzed with data from 20-gallon traps for one year in the orchard of the hybrid 'Enano-Verde de Brasil’. This type of trap, due to its easy elaboration, is the most used by coconut producers in the region. Trapping R. palmarum adults in ‘Alto Pacifico ecotype 2’ and ‘Enano-Verde de Brasil’ were compared based on data from six-gallon traps from each orchard. Each trap was considered as a replicate. Trapping data were collected for 52 weeks, and total insect trapping, and number of females and males were recorded.
Estimate of the annual trapping cost and its cost-benefit.
The annual trapping cost (ATC) of each of the traps used was calculated using the equation proposed by Murguía-González et al. (2017):
where,
Ct = cost of each trap based on the cost of the materials for manufacture
Cf = cost of the aggregation pheromone in the year under study
Frf = frequency of pheromone replacement (every three months)
Cmol = cost of molasses according to the price of the livestock association of Tecomán, Colima
Cc = cost of sugarcane in the local market.
FrCb = frequency of feed bait replacement (every 15 days)
Cmu = cost of weekly sampling obtained by dividing one minimum wage by the six repetitions of each type of trap.
Fm = frequency of sampling in one year
Nt = number of traps (replicates) used for each trap type.
The cost-benefit of trapping R. palmarum, per trap type, was determined by dividing the ATC by the total number of adults trapped.
Data analysis
The response variable (trapping) to determine trap efficiency was analyzed using a deviancy analysis with a quasi-Poisson distribution (Crawley, 2012). This analysis is a generalized linear model (GLM) that analyzes one or more discrete independent variables (such as treatments) and a numerical dependent variable, such as insect trapping, in this case, discrete because they were counted. Subsequently, means were compared with the least significant difference test (P ≤ 0.05). A Spearman correlation (P ≤ 0.05) was made between insect trapping and temperature (mean, minimum and maximum), relative humidity, precipitation and wind speed. The environmental parameters of the two coconut orchards were obtained from the weather station in Armería (18.9146, -103.9692) and Tecomán (18.9668, -103.8422) of the Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP) in Colima. All analyses were performed with the program ‘R’ (R Core Team, 2016).
Results
Trap efficiency
Table 1 shows the trapping in 52 weeks of sampling in a plantation of C. nucifera ‘Alto Pacífico ecotipo 2’ (Figure 2). A total of 3 414 insects were trapped (2 144 females = 63 % and 1 270 males = 37 %) with a female:male ratio of 1.7:1. According to statistical analyses (F = 4.08, P = 0.014), the most effective traps for capturing R. palmarum were the trash can and CSAT traps with average of 229 and 186 adults captured per trap, which represented 40.3 % and 32.7 % of the total, respectively. In the case of males and females, the same behavior was found for trash can traps (82.66 males and 146.83 females) and CSAT (68.66 males and 117.50 females), which had the highest trapping values (P < 0.05) compared to the other traps.
Table 1 Trapping Rhynchophorus palmarum adults in five types of traps in a coconut orchard with the genotype 'Alto Pacífico ecotipo 2' in one year of monitoring in Armería, Colima, Mexico.
| Type of trap | Males | Females | Males and females | Females: males ratio | Trapping (%) | |||
|---|---|---|---|---|---|---|---|---|
| Per trap | Total | Per trap | Total | Per trap | Total | |||
| Bucket | 26.33 ± 1.6 bc | 158 | 38.66 ± 2.1 c | 232 | 65.00 ± 3.4 b | 390 | 1.4:1 | 11.4 |
| Trash can | 82.66 ± 16.1 a | 496 | 146.83 ± 23.9 a | 881 | 229.50 ± 40.0 a | 1 377 | 1.7:1 | 40.3 |
| Gallon | 20.33 ± 0.9 c | 122 | 35.83 ± 1.6 c | 215 | 56.16 ± 2.3 b | 337 | 1.7:1 | 9.9 |
| CSAT | 68.66 ± 5.3 b | 412 | 117.50 ± 7.9 b | 705 | 186.16 ± 13.1 a | 1 117 | 1.7:1 | 32.7 |
| Bottle | 13.66 ± 1.0 c | 82 | 18.50 ± 1.6 c | 111 | 32.16 ± 2.6 b | 193 | 1.3:1 | 5.7 |
| Total trapping | 1 270 | - | 2 144 | - | 3 414 | - | 1.7:1 | 100 |
| F value | 3.09 | - | 4.77 | - | 4.08 | - | - | - |
| P value | 0.039 | - | <0.007 | - | <0.014 | - | - | - |
CSAT = Colegio Superior de Agricultura Tropical trap. ± Standard deviation of the mean. Letters in each column indicate grouping of means according to the least significant difference (P ≤ 0.05).
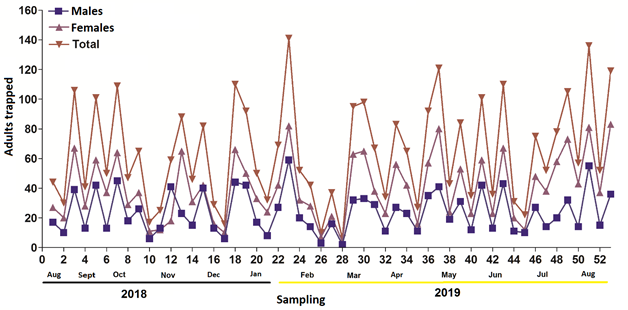
Figure 2 Fluctuation in trapping Rhynchophorus palmarum with five types of traps for one year in a coconut orchard with the genotype 'Alto Pacífico ecotipo 2' in Armería, Colima, Mexico.
Figure 3 shows the capture fluctuation of R. palmarum with the gallon trap in the orchard with the hybrid ‘Enano-Verde de Brasil’. During the sampling year, 4 799 adults were captured, of which 1 725 were males (36 %) and 3 074 females (64 %), with a ratio of 1.7:1 females:males, respectively. Figure 4 compares the two coconut plantations; according to Table 2, there were statistically significant differences in male (F = 59.36, P < 2 x 10-11), female (F = 67.36, P < 2 x 10-11) and total (F = 71.40, P < 7 x 10-13) capture.

Figure 3 Fluctuation in trapping Rhynchophorus palmarum using gallon traps for one year in a coconut orchard with the genotype ‘Enano-Verde de Brasil’ in Caleras, Tecomán, Colima, Mexico.
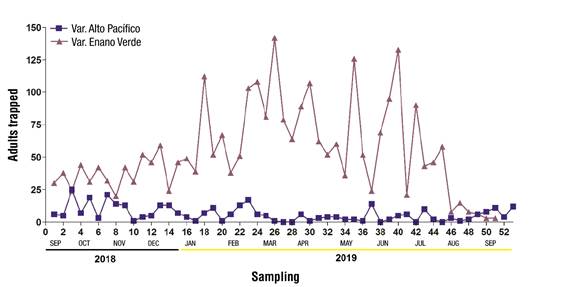
Figure 4 Comparison of Rhynchophorus palmarum captured with gallon traps in two coconut plantations with genotypes ‘Alto Pacífico ecotipo 2’ and ‘Enano-Verde de Brasil’ for one year of monitoring in Armería, Colima, Mexico.
Table 2 Deviance analysis with quasi-Poisson distribution in the comparison of Rhynchophorus palmarum capture with gallon traps in two coconut orchards for one year of monitoring in Armería, Colima, Mexico.
| Genotype | Total | Males | Females |
|---|---|---|---|
| Alto Pacífico | 6.49 ± 4.89 | 2.31 ± 0.73 | 4.17 ± 2.49 |
| Enano-Verde de Brasil | 29.60 ± 50.96 | 10.70 ± 8.24 | 18.89 ± 21.10 |
| F value | 71.4 | 59.36 | 67.36 |
| P value | <7 x 10-13 | 2 x 10-11 | 2 x 10-11 |
±Standard deviation of the mean.
Cost-benefit of trapping Rhynchophorus palmarum
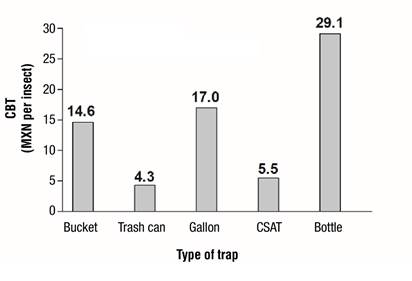
CSAT and trash can traps had the highest annual cost, while the bottle trap was the least expensive, due to the material used (Table 3); however, when including insect capture in the cost-benefit equation (Figure 5), it was observed that TCT and CSAT had the best cost-benefit with 4.3 and 5.5 MXN per insect, respectively. In contrast, the bottle trap had the highest capture cost per insect (29.1 MXN).
Table 3 Annual cost of mass trapping of Rhynchophorus palmarum in a coconut orchard with the genotype 'Alto Pacífico ecotipo 2' in Armería, Colima.
| Trap | Cebo | Pheromone | Sampling | Annual cost (MXN) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Type | Cost (MXN) | Molasses (MXN) | Sugar cane (MXN) | Replacements | Cost (MXN) | Replacements | Cost* (MXN) | Number | Per trap | Total (n = 6) |
| Bucket | 12 | 1.5 | 0.2 | 24 | 55 | 4 | 14.08 | 48 | 948.64 | 5 691.84 |
| Trash can | 49.5 | 1.5 | 0.2 | 24 | 55 | 4 | 14.08 | 48 | 986.14 | 5 916.84 |
| Gallon | 20 | 1.5 | 0.2 | 24 | 55 | 4 | 14.08 | 48 | 956.64 | 5 739.84 |
| CSAT | 85 | 1.5 | 0 .2 | 24 | 55 | 4 | 14.08 | 48 | 1 021.64 | 6 129.84 |
| Bottle | 0 | 1.5 | 0.2 | 24 | 55 | 4 | 14.08 | 48 | 936.64 | 5 619.84 |
CSAT = Colegio Superior de Agricultura Tropical trap. *The cost of weekly sampling was determined by dividing one minimum wage by the six replicates of each trap.
Correlation between Rhynchophorus palmarum trapping and climate variables
The climate factors correlated with insect capture in the 'Alto Pacífico ecotype 2' plantation were relative humidity (rho = -0.2814, P = 0.0412) with total capture; maximum temperature (rho = 0.2995, P = 0.0293) and, again, relative humidity (rho = -0.2929, P = 0.0332) with the capture of females (Table 4). It is important to mention that male capture was not correlated with climate variables. In the case of the orchard with the hybrid ‘Enano-Verde de Brasil’ (Table 5), total capture was correlated with minimum temperature (rho = -0.4477, P = 0.0011), average temperature (rho = -0.3862, P = 0.0056) and relative humidity (rho = -0.3650, P = 0.0091). Male capture was correlated with accumulated precipitation (rho = -0.3016, P = 0.0332), average precipitation (rho = -0.2987, P = 0.0350), minimum temperature (rho = -0.552753, P = 3 x 10-5), average temperature (rho = -0.4930, P = 0.0002) and relative humidity (rho = -0.4292, P = 0.0018). Female capture was correlated with minimum temperature (rho = -0.3608, P = 0.0100), average temperature (rho = -0.2922, P = 0.0394) and relative humidity (rho = -0.3306, P = 0.0190). Correlations in both orchards were weak, less than 50 %.
Table 4 Spearman correlation coefficient between climate variables and Rhynchophorus palmarum trapped in a coconut orchard 'Alto Pacífico ecotipo 2' in Armería, Colima, Mexico.
| Climate variable | Total capture | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| rho | P value | α = 0.05 | rho | P value | α = 0.05 | rho | P value | α = 0.05 | |
| Accumulated precipitation (mm) | -0.0257 | 0.8549 | NS | -0.0956 | 0.4957 | NS | 0.0310 | 0.8252 | NS |
| Average precipitation (mm) | -0.0257 | 0.8549 | NS | -0.0956 | 0.4957 | NS | 0.0310 | 0.8252 | NS |
| Maximum temperature (°C) | 0.2661 | 0.054 | NS | 0.1529 | 0.2743 | NS | 0.2995 | 0.0293 | * |
| Minimum temperature (°C) | 0.0726 | 0.6051 | NS | -0.0038 | 0.978 | NS | 0.1140 | 0.4159 | NS |
| Average temperature (°C) | 0.1245 | 0.3742 | NS | 0.0403 | 0.7743 | NS | 0.1617 | 0.2471 | NS |
| Wind speed (km∙h-1) | 0.0292 | 0.8354 | NS | -0.0117 | 0.9337 | NS | 0.0414 | 0.7684 | NS |
| Relative humidity (%) | -0.281417 | 0.0412 | * | -0.2247 | 0.1057 | NS | -0.2929 | 0.0332 | * |
Non-significant (NS) and significant (*).
Table 5 Spearman correlation coefficient between climate variables and Rhynchophorus palmarum captured in a coconut orchard with the hybrid ‘Enano-Verde de Brasil’ in Caleras, Tecomán, Colima, Mexico.
| Climate variable | Total capture | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| rho | P value | α = 0.05 | rho | P value | α = 0.05 | rho | P value | α = 0.05 | |
| Accumulated precipitation (mm) | -0.1259 | 0.3834 | NS | -0.3016 | 0.0332 | * | -0.0677 | 0.6403 | NS |
| Average precipitation (mm) | -0.1272 | 0.3784 | NS | -0.2987 | 0.0350 | * | -0.0703 | 0.6271 | NS |
| Maximum temperature (°C) | 0.1723 | 0.2313 | NS | 0.1128 | 0.4353 | NS | 0.1945 | 0.1757 | NS |
| Minimum temperature (°C) | -0.4477 | 0.0011 | ** | -0.5527 | 0.00003 | *** | -0.3608 | 0.0100 | * |
| Average temperature (°C) | -0.3862 | 0.0056 | ** | -0.4930 | 0.0002 | *** | -0.2922 | 0.0394 | * |
| Wind speed (km∙h-1) | 0.1632 | 0.2574 | NS | 0.0067 | 0.9629 | NS | 0.2415 | 0.0909 | NS |
| Relative humidity (%) | -0.365 | 0.0091 | ** | -0.4292 | 0.0018 | ** | -0.3306 | 0.019 | * |
Non-significant (NS), significant (*), highly significant (**) and highly significant (***) correlations.
Discussion
Efficiency of traps for capturing Rhynchophorus palmarum
The trash can and CSAT trap were the most effective, trapping 6.13 and 4.78 times more R. palmarum adults, respectively, compared to the bottle trap which was the least effective. In another study, Ovando-Cruz et al. (2019) compared the CSAT trap against the yellow PET (polyethylene terephthalate) type and found that the former was more effective by capturing 0.33 times more insects. Murguía-González et al. (2017) evaluated the efficiency of the yellow bucket trap (similar to CSAT) with the white bucket, clear bottle, and green bottle types (same as the one evaluated in this study). The yellow bucket trap captured 1.22 times more insects than the green bottle trap; this result is similar to that found in the present study, since the CSAT trap captured 4.78 times more insects than the bottle type.
On the other hand, Sumano et al. (2012) evaluated bucket traps with variants in their holes and compared them with the CSAT trap. The non-mesh bucket trap was the most effective, capturing 0.20 times more insects than the CSAT trap, which differs with the results of the present study, as it captured 1.86 times more insects than the bucket type. This discrepancy could be due to the height and arrangement of the traps in the field, a factor that should be considered in future studies.
Mechanical control, through trapping, has been shown to be effective in controlling R. palmarum populations by reducing the number of affected palms (Alpizar et al., 2009; Miguens et al., 2011). Hence the importance of evaluating and finding the most effective trap designs at the lowest cost. On the contrary, traps that do not show significant differences in capture have been reported. In this regard, Miguens et al. (2011) evaluated bucket and PET (soft drink) bottle traps and indicated there were no significant differences in insect capture, possibly because they did not use the aggregation pheromone. On the other hand, Montes and Ruiz (2014) used 20 L gallons with the same characteristics as those used in the present study; the variant was with and without plastic dispenser (600 mL) for the food bait (molasses), but no significant differences were found in the captures of R. palmarum.
Milosavljević, Hoddle, Mafra-Neto, Gómez-Marco, and Hoddle (2020) evaluated the bucket trap against the Picusan® trap, imported from Europe; the latter trapped five times more insects. This could be due to the position of the trap, because Picusan® was placed on the ground, where the insect can walk and climb to enter the trap, while the bucket type was hung 1 m high, which could make it difficult for the insect to enter, as it has a wandering flight. Recently, Milosavljević, Hoddle, Mafra-Neto, Gómez-Marco, and Hoddle (2021) concluded that bucket traps suspended 1.5 m above the ground attracted 30 % more insects than Picusan® traps placed on the ground; however, 82 % of the insects that entered the former escaped, while Picusan® retained 89 %. The result could be due to the design and color, because the latter is more airtight and black in color; usually, R. palmarum shelters in the dark.
Regarding the capture of males and females, in the two experimental plots, the capture of females was higher for all treatments, which coincides with that mentioned by Milosavljević et al. (2020), Moya-Murillo et al. (2015) and Ferreira et al. (2003), who attribute it to the use of aggregation pheromone; however, this is not a general rule, because other studies on aggregation pheromone disagree (Montes & Ruiz, 2014; Murguía-González et al., 2017; Sumano et al., 2012). It is possible that the capture of more females than males is because in this study no insecticides were used in the traps; therefore, males remained alive, probably generated their own pheromones and attracted more females as mentioned by Rodríguez-Currea et al. (2017).
No comparative studies of R. palmarum capture between coconut genotypes have been carried out; therefore, the present study represents the first contribution of comparison in two orchards with ‘Alto Pacifico ecotype 2’ (30-year-old palms and replants less than five years old) and ‘Enano-Verde de Brasil’ (two years old approximately). Carreño-Correa et al. (2013) point out that the age of the crop is not a determining factor in the incidence of the pest, although in the present study it was. On the other hand, there is agreement with Landero-Torres et al. (2015), who suggest that the morphology of the palm influences incidence; the genotype ‘Enano-Verde de Brasil’ is short, early and uniform in flowering compared to ‘Alto Pacífico ecotipo 2’ that can measure up to 15 m in height, which possibly favored a higher incidence and capture in the first genotype during the experimental period. Furthermore, Alpizar et al. (2002) and Fernandes et al. (2020) mention that agronomic management also influences pest incidence, since damaged, diseased or stressed palms release volatiles such as ethyl acetate acting as a natural attractant for R. palmarum (Moya-Murillo et al., 2015).
Cost-benefit of tramping Rhynchophorus palmarum
In mass trapping studies, costs are calculated based on labor, construction materials, food baits, pheromones and even insecticides, looking for the least expensive option. Montes and Ruiz (2014) calculated costs based on labor, materials and equipment; their results indicate that the molasses dispenser trap has an annual cost of 98.160 COP (Colombian pesos), while the one without dispenser is 112.85 COP. In another study, Murguía-González et al. (2017) reported that the colorless and green bottle traps were the least expensive (804 USD∙ha-1) followed by bucket (831 USD∙ha-1). However, in these cases they did not calculate the cost-benefit of trapping, whereas in the present study they did, because it is important for decision making regarding the trap choice.
Correlation between Rhynchophorus palmarum capture and climate variables
The environmental variable with the highest correlation with captures of R. palmarum in the two coconut plantations was relative humidity. The correlation was negative; that is, the lower the relative humidity, the higher the captures. Maximum temperature was only positively, but weakly, correlated with the capture of females in the ‘Alto Pacífico ecotype 2’ plantation. In the orchard with the hybrid ‘Enano-Verde de Brasil’, minimum and average temperature were negatively correlated with all trapping, while accumulated and average precipitation were only negatively correlated with male capture. This may be due to the low climate variability in the study area, which was reflected in similar insect captures throughout the year. However, the results also indicate that the correlations are weak. No clear trend in the effects of climate factors with the incidence of this insect has been observed in other studies. Cysne et al. (2013) carried out an experiment in the western Amazon region of Brazil, with a tropical monsoon climate and annual thermal oscillation of less than 5 °C and frequent precipitation. The results showed no significant correlations, attributing it to the regularity of rainfall, little variation in humidity and temperature. In the northern zone of Rio de Janeiro, Brazil, with a warm tropical sub-humid climate, relatively low rainfall (734 mm per year) and high temperatures (26 °C annual average), Miguens et al. (2011) found no significant differences in the abundance of the insect; the site condition was similar to that of the present work. Also, Montes and Ruiz (2014) observed no correlation between insect capture and precipitation, in Santander, Colombia, with tropical warm rainy climate, mean annual temperature of 28 °C and mean annual precipitation of 2 836 mm.
On the other hand, Milosavljević et al. (2020) and Pinho et al. (2016) mention that trapping increased in the months of lower precipitation as was the case of male capture in the plantation ‘Enano-Verde de Brasil’; in contrast, Murguía-González et al. (2017), Correia et al. (2015), Landero-Torres et al. (2015) and Ferreira et al. (2003) found that R. palmarum is more abundant in the season of higher precipitation. Finally, in banana plantations, Takada, Batista, Hojo, and Carvalho (2011) concluded that the population of R. palmarum increased during periods of high temperatures as in the ‘Alto Pacifico’ plantation with female capture, as opposed to periods of low precipitation where capture is significantly reduced.
Relevance of the study
The efficacy of the traps and the cost-benefit for trapping R. palmarum allow determining which type of trap should be use for mechanical and ethological control (use of aggregation pheromone, Murguía-González et al., 2017). The elaboration of homemade traps such as those evaluated in this research and the use of aggregation pheromone represent an economic strategy if compared to the weekly or biweekly application of chemical insecticides (carbaryl, carbofuran, fipronil, imidacloprid and phenylpyrazole) for the control of R. palmarum (Abdelazim et al., 2017); it also reduces environmental pollution by the constant application of insecticides and contributes with the safety of the fruit and export derivatives. Trapping with aggregation pheromone has become the most accepted strategy by organic and inorganic coconut producers of local and national companies, so that the present study contributes to the definition of the type of trap used for the capture of R. palmarum.
Conclusions
The most effective traps, statistically, for mass trapping of Rhynchophorus palmarum were trash can and CSAT (Colegio Superior de Agricultura Tropical trap); these had the best cost-benefit with 4.3 MXN and 5.5 MXN per insect, respectively. In the coconut orchard with the hybrid ‘Enano-Verde de Brasil' (younger age, low and early growth) 3.56 times more insects were captured compared to the orchard of 'Alto Pacífico ecotipo 2', when using the same type of trap and the same number of replicates. Humidity showed a weak and negative correlation with the capture of R. palmarum for the two coconut genotypes. The effectiveness of the traps and the cost-benefit for capturing R. palmarum will allow producers to determine which trap to use for mechanical and ethological control (aggregation pheromone).




![Variación fitoquímica entre procedencias de oyamel (Abies religiosa [Kunth] Schltdl. & Cham.) en un gradiente altitudinal](/img/es/prev.gif)






 texto en
texto en