Highlights:
The pest belonging to the genus Zadiprion, pinyon pine, was detected at the species level.
The lancet of the ovipositor has nine rings: the first one with an inverted “U” shape.
The sawfly under study was recorded as Zadiprion borjai sp. nov. (GenBank: ON181557).
Z. borjai sp. nov. shows morphological differences with Z. jeffreyi and Z. rohweri.
Z. borjai sp. nov. do not affect Pinus nelsonii species in Miquihuana, Tamaulipas.
Introduction
Sawflies (Hymenoptera: Diprionidae) affect trees of the genera Pinus, Picea, Pseudotsuga and Abies in temperate coniferous forests in the United States of America, Mexico and Guatemala (Smith, 1988); they are considered important forest pests because they cause defoliations. Sometimes they reach epidemic levels and after repeated defoliations cause the death of trees in hundreds or thousands of hectares of forest. Mexico reports the genera Zadiprion, Neodiprion and Monoctenus, being Zadiprion the most numerous in species and with the widest distribution in the country.
Zadiprion Rohwer (Hymenoptera: Diprionidae: Diprioninae) is a group that originally comprised five species, although it was mentioned that the fauna of Mexico was not precisely known. In 2012, Zadiprion ojedae (Smith and Sánchez-Martínez) was added attacking Pinus durangensis (Martínez) in Chihuahua, Mexico (Smith, Sánchez-Martínez, & Ojeda-Aguilera, 2012). In 2019, Zadiprion jeffreyi (Smith) was described as a new species attacking Pinus jeffreyi Balf. in Parque Nacional San Pedro Mártir in Baja California, Mexico (Aguilera-Molina, Munguía-Ortega, López-Reyes, Martínez-Aquino, & Ceccarelli, 2019; Smith, 2019). The latter is closely related to Zadiprion rohweri (Middleton) considered a pest of pinyon pines characterized by an inverted “U” on the first lancet ring of the female ovipositor near the dorsum. However, larvae differ in mating and host (Aguilera-Molina et al., 2019; Smith, 2019).
In the Sierra de Miquihuana, Tamaulipas, Mexico, sawfly attacks were reported on Pinus cembroides (Zucc.) and the causal species was initially identified as Z. rohweri, due to the characteristics of the ovipositor lancet; however, upon close examination of the larvae, very noticeable differences were observed from both Z. rohweri from Coahuila (Smith, Monjarás-Barrera, Aguilar-Hernández, & Quiñones-Dena, 2016) and Z. jeffreyi from Baja California (Aguilera-Molina et al., 2019).
Therefore, it is necessary to corroborate whether the species that is attacking P. cembroides in Tamaulipas is a new species or an already reported species. This study shows information on the morphological description of larvae, adults and ovipositor of the female sawfly that attacks pinyon pine.
Materials and Methods
Study area
The study was carried out in "Mesa del Jarrillo", ejido Servando Canales in Miquihuana, Tamaulipas (23° 38’ 39.2” N, 99° 50’ 37.2” W; 2 210 m). The predominant climate is Aw1, warm sub-humid with rain in summer, mean annual temperature higher than 22 °C and temperature of the coldest month higher than 18 °C; vegetation is composed of thorny scrub with predominant species such as Dasylirion miquihuanensis Bogler, D. berlandieri Watson, Leucophyllum frutescens [Berland] I. M. Johnston, Pinus nelsonii Shaw and oaks (Quercus spp.); the topography consists of mountains and hills and the soils are predominantly Lithosol and Xerosol (Gobierno de Tamaulipas, 2011).
On November 22, 2017, third instar larvae were collected. These were transferred in cool containers, with foliage of the host pine (P. cembroides), to the Forestry and Agricultural Health Laboratory at Campo Experimental Pabellón (CEPAB) in Aguascalientes. One group of larvae was used to complete the biological cycle and thus obtain new adults, another group was used for morphological identification and a third group for molecular identification.
From the group of larvae used to complete the biological cycle, cocoons were obtained. These were placed in 250 mL plastic containers with organza cover and placed in a Binder 720 KBW E5.1 chamber at a controlled temperature of 26 ± 2 °C with a photoperiod of 14:10 light and dark, respectively. Containers with pupae were monitored three times a week to record adult emergence.
Morphological identification of Zadiprion sp. larvae
Larvae were killed by immersing them in hot water at 70 °C for 3 min and then transferred to 70 % (v/v) alcohol (González et al., 2014). Larvae were described based on the writings of Ross (1955), Yuasa (1922), Wong and Szlabey (1986), and González et al. (2014) with the support of images taken with a cell camera through the objective of a Motic dissecting microscope at 40X magnification.
Morphological identification of Zadiprion sp. adults
Adults collected from cocoons kept under laboratory conditions were identified based on the descriptions of Smith et al. (2012), Smith (2019) and with the terminology of Goulet (1992).
The ovipositor lancet of the females and the genitalia of the male were removed after a cold maceration process. The abdomen was separated from the body and introduced into a 4 mL Eppendorf tube with 10 % KOH for 24 h. At the end of maceration, the abdomen was rinsed with distilled water and dehydrated in alcohol. The structures were temporarily mounted in glycerin and photographs were taken with a compound microscope (Motic BA 200); the female lancet was also photographed with a scanning electron microscope (ZEISS EVO® MA 15).
Molecular identification of Zadiprion sp.
DNA was extracted from a group of eight larvae in 96 % anhydrous alcohol. They were washed three times with sterile distilled water and placed in 1.5 mL Eppendorf tubes, where liquid nitrogen for maceration and 500 μL of lysis buffer were added. The tube rested at room temperature for 30 min and was subsequently centrifuged for one minute at 12 000 rpm. The supernatant was recovered in a new tube, adding 300 μL of phenol: chloroform (1:1), vortexed for 1 min, centrifuged at 12 000 rpm for 3 min. The new supernatant was transferred to another tube and 200 μL of phenol: chloroform (1:1) was added, then transferred to a new tube and 300 μL of isopropanol and 30 μL of ammonium acetate were added. The mixture was centrifuged for 5 min at 12 000 rpm; the supernatant was decanted, and the pellet was washed with 50 μL of 70 % ethanol. The precipitate was dried at room temperature and the pellet was resuspended in 50 μL of nuclease-free water. DNA quality was measured on a NanoDrop™ 2000 (Thermo Scientific). The product was stored at 4 °C.
Universal primers LCO1490 (5-GGTCAACAAATCATAAAGATATTGG-3´) and HCO2198 (5´TAAACTTCAGGGTGACCAAAAAATCA-3´) (Folmer, Nilges, Folkers, Konings, & Hilbers, 1994) were used for amplification of ribosomal DNA regions, which recognize the Cytochrome Oxidase, Subunit I (COI) gene. PCR reactions were performed by addition of the reagents: PCR buffer (5X: 5.0 µL, concentration:amount respectively) MgCl2 (25 nM: 0.75 µL), dNTP´s (10 nM: 0.25 µL), primer 1 (10 nmol: 1.0 µL), primer 2 (10 nmol: 1.0 µL), DNA Taq polymerase (5U∙µL-1: 0.5 µL), blank DNA (20 ng∙µL-1: 3.0 µL) and 13.5 µL of PCR grade water.
Amplification was performed on a thermal cycler (T100TM Thermal Cycler, Bio-Rad) with the following program: 1) initial denaturation 1 cycle of 3 min at 95 °C; 2) denaturation 1 min at 95 °C; 3) alignment five cycles of 30 s at 51 °C; 4) extension 1 min at 70 °C, 5) denaturation 1 min at 94 °C, 6) alignment 35 cycles of 30 s at 51 °C, 7) extension 1 min at 72 °C, 8) final extension one cycle of 5 min at 72 °C and 9) storage at 4. 0 °C. The amplified fragments were run on 1 % agarose gels with 0.4 μL of ethidium bromide and visualized on a photodocumenter (Infinity-ST5 VILBER Lourmat). The band of interest was cut for purification with the Wizard® SV Gel and PCR Clean-Up System kit following the supplier's protocol (Promega Corporation, 1999). Fifty µL of solution 1 was added to the agarose gel preserved in Eppendorf tubes and dissolved at 65 °C. The diluted gel mix was transferred to a minicolumn inserted into a collecting tube, incubated at room temperature for 1 min, centrifuged at 14 000 rpm for 1 min and the supernatant was decanted. Subsequently, two washes were performed with 700 µL of solution 2, centrifuged at 14 000 rpm for 3 min and the supernatant was decanted from the collecting tube. The minicolumn was transferred to a sterile Eppendorf tube and dried at room temperature. Fifty µL of nuclease-free water were added, incubated at room temperature for 5 min and centrifuged at 14 000 rpm for 1 min; the minicolumn was discarded and the product was stored ± 4 °C. The purified PCR products were sent for sequencing to Macrogen Korea in Seoul, Republic of Korea.
The sequences were assembled in the SeqMan Pro module of the DNASTAR Lasergene Molecular Biology 16 program. Each assembly was submitted to BLAST (Basic Local Alignment Search Tool) homology analysis in the nucleotide database of the NCBI (National Center of Biotechnology Information, 2021) and analyzed in the BOLDSYSTEMS database (2021).
A phylogenetic analysis was carried out for Zadiprion where the sequencing assemblages of the collected specimens and the reference databases resulting from the Gen Bank search were included. Sequences were aligned using the clustering method with the CLUSTAL OMEGA 1.2.2 program (Sievers & Higgins, 2014) and the best nucleotide substitution model was searched with the ModelTest-NG program (Darriba et al., 2019). Phylogenetic reconstruction was performed with Bayesian inference using Markov Chains Monte Carlo (MCMC), implemented in the BEAST v1.10.4 program (Suchard et al., 2018) with 1 000 000 generations. It should be mentioned that the GenBank sequence HM114320, corresponding to Sirex noctilio Fabricius 1793, was used as an outgroup control (Hymenoptera: Siricidae).
Results and Discussion
Zadiprion borjai González et al., 2021 sp. nov.
Female
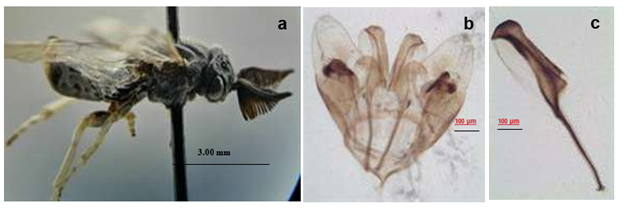
The female measures 9.3 mm in length, is light yellow with dark sutures on the thorax, has a yellow head with dark triangular spots around the ocelli, two dark longitudinal lines behind the lateral ocelli that continue to the occiput and presents a great number of long yellow hairs on the head. In frontal view, the head has a yellowish appearance with tentorial holes; the margins and apex of the clypeus are dark, and the mandibles are brown. The antennae are serrate with 20 light brown antennomeres and the apical antennomeres are dark brown. The thorax is yellowish, the median lobe of the mesoscutum has a light brown spot on the anterior portion and the central keel is dark brown. It has lateral lobes, each with two dark spots in parallel longitudinal lines with straight, but irregularly shaped sides. The mesoscutellum is yellowish white highlighted by dark sutures on the thorax. The mesopleuron is yellow with a light brown triangular spot on the mesial side; the margins and apex are dark. The wings are yellowish brown with brown stigma with two hyaline areas. The abdomen, in dorsal view, is yellowish with the junction of the tergites slightly brown; the basal segments have a larger brown area. The legs are yellowish with fine pubescence, the femurs have a brown inner portion, the tibiae have a yellowish basal third and the rest is brown like the tarsi (Figure 1).

Figure 1 Female of Zadiprion borjai sp. nov. attacking Pinus cembroides in Miquihuana Tamaulipas, Mexico. Perched on host (a), dorsal view (b) and lateral view (c).
The lancet of the ovipositor (Figure 2) has nine rings; the first ring is shaped like an inverted “U”. The teeth of the first complete ring are larger at the base, the first three serrulate are concave in the mesial part and the rest of the rings are straight.
Males
Males are 6.0 mm in length and look dark black with a yellow spot in the postocular area near the upper margin of each eye. They have bipectinate antennae except for the three terminal segments. The base of the antennomeres is light brown with black ramus. The face is almost entirely black with yellowish hairs. The labrum is light yellow; the mandibles are brown, and the palps are yellowish. The coxae and trochanters of the legs are black, the femurs black with yellow apical third, and yellowish tibiae and tarsi. The genitalia show rounded and clear harps at the tip with the inner part slightly curved and concave at the center (Figure 3b). The penis valve shows the apex straight in the front portion and rounded convex in the rest with two triangular projections in the middle part (Figure 3c).
In the case of smaller flies, the dark spot surrounding the ocelli is wider with no light portions between the ocelli and the spots on the lateral lobes of the mesoscutum merge and form a continuous dark line longitudinally.
Type material
Dry-mounted female holotype labeled Miquihuana, Tamaulipas, Mexico. "Mesa del Jarrillo” 23° 38’ 39.2” N - 99° 50’ 37.2” W and altitude of 2 210 m. Ernesto Gonzalez Gaona collector of March 16, 2019. Dry-mounted male with the same data as above. These specimens were deposited with record number INIT-1495 in the forest insect collection of the Centro Nacional de Investigación Disciplinaria en Conservación y Mejoramiento de Ecosistemas Forestales del Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (CENID-COMEF-INIFAP), Viveros de Coyoacán, Mexico City.
Paratype
Three females and three males with the same collection data with record numbers 245 and 246, respectively, deposited in the forest insect collection of CEPAB-INIFAP, km 32.5 Aguascalientes-Zacatecas highway, Pabellón de Arteaga, Aguascalientes, Mexico.
Etymology
The name Zadiprion borjai has been assigned in honor of Hugo Enrique Borja Nava (health liaison of the Comisión Nacional Forestal [CONAFOR]), who in 2013 detected the epidemic outbreak attacking P. cembroides in the ejido Servando Canales, Miquihuana, Tamaulipas, Mexico, and has followed up on the study and phenology of the pest.
Material studied
Miquihuana, Tamaulipas, Mexico. “Mesa del Jarrillo” of the ejido Servando Canales (23° 38’ 39.2” N, 99° 50’ 37.2” W and elevation of 2 210 m). Infestation in a 12-year-old commercial production lot of P. cembroides. This type of plantation is carried out with the objective of having a source of economic income in low-income areas, which is why CONAFOR has encouraged planting, since the seed of P. cembroides is marketed in preference to P. nelsonii Shaw, which is considered a hard pine nut. In the area, P. nelsonii is found naturally and no attacks have been recorded.
Comments
In general, Zadiprion females are very similar since they are yellow. The species under study is closely related to Z. jeffreyi and Z. rohweri because the lancet of the ovipositor also has the first inverted U-ring (Smith et al., 2012). This suggests that Z. rohweri is not the only species with this characteristic, as mentioned by Smith et al. (2012), regarding the three species as a group. In this case, a noticeable difference is observed in the lateral lobes of the mesonotum: Zadiprion borjai sp. nov. has two lines of parallel longitudinal dark spots with straight but irregularly shaped sides, while Z. jeffreyi has a black spot (Aguilera-Molina et al., 2019; Smith, 2019) and Z. rohweri is all-yellow with no stain (Smith et al., 2012). The lancet most closely resembles that of Z. jeffreyi (Smith, 2019) in being short and robust, but Z. borjai has nine rings and rings two, three and four are slightly curved: the first one with larger base teeth. Z. borjai males are preponderantly black with yellowish legs and show a yellowish-brown spot in the postocellar area near the upper orbit of the compound eye, but no yellowish-brown pronotum or tegula as Z. rohweri. Likewise, Z. borjai differs in the genitalia, as it has rounded, clear harps at the apex and in the other cases it is a bit sharp; the penis valve also differs from the other two species at the apex.
At the larval level, this group of insects has a very distinctive maculation and can be separated at this stage. Larvae affecting P. cembroides in Miquihuana, Tamaulipas (Figure 4c) are different from those attacking P. jeffreyi in Baja California, Mexico, identified as Z. jeffreyi (Figure 4b) (Aguilera-Molina et al., 2019), and from Z. rohweri attacking P. cembroides in Coahuila, Mexico (Figure 4a) (Smith et al., 2016).
Zadiprion borjai larvae are light with dark longitudinal stripes without glandubules in clusters. Viewed dorsally they have a brown longitudinal spot with two thin white lines on the sides and a wide dark longitudinal stripe subdorsally. The head is orange-brown with a small circular eyelid. From the lateral view, and only on the abdominal segments, they show a broad dark spot extending from below the spiracle to the base of the false legs, leaving only the areas of the prepipleurite lobes light (Figure 4c).

Figure 4 Zadiprion larvae of the Z. rohweri group affecting pines in Mexico: Z. rohweri in Pinus cembroides in Coahuila (a), Z. jeffreyi in P. jeffreyi in Baja California (b), Z. borjai sp. nov. in P. cembroides in Tamaulipas (c), Zadiprion sp. in P. chiapensis (d).
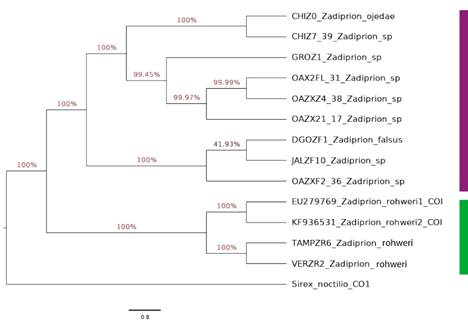
Regarding molecular identification, in the NCBI taxonomy databases, the genus Zadiprion has only four sequences recorded that correspond to COI, and of these only two were identified to species as Z. rohweri.
A phylogenetic tree (Figure 5) with several clades was obtained. Homology analysis with BLAST and BOLDSYSTEMS placed the samples from Miquihuana, Tamaulipas (TAMPZR6) as different species from the populations of the specimens recorded in GenBank (ID KF936531) with 92.23 % and 93.72 % similarity, respectively, corresponding to Z. rohweri.
The sequence of the new Zadiprion species was recorded in GenBank under deposit number ON181557 dated 12 April 2022.
Conclusions
The pest that is affecting pinyon pine (Pinus cembroides) is different from Z. rohweri and Z. jeffreyi. The species was recorded as Zadiprion borjai sp. nov. (deposited in GenBank No. ON181557) with known distribution only in Miquihuana, Tamaulipas. In this region, P. cembroides has a better price than P. nelsonii; therefore, the identification of the pest is important considering that the planting of pinyon pine is being promoted to increase the resources of producers.











 texto en
texto en