Highlights:
The effect of pre-germinative treatments and vegetative propagation methods on A. pungens was analyzed.
Germination was higher (73.33 %) by scarification with H2SO4 for five and six hours.
Heat shock (100 °C) and cold stratification (4 °C) caused low or no germination.
Root formation was achieved in 37.5 % of air layering, but not in semi-lignified cuttings.
Introduction
Temperate forests in Mexico are mainly distributed in mountainous areas and represent 16.56 % of the country's surface area, which includes coniferous and oak forests (Challenger & Soberón, 2008; Instituto Nacional de Estadística y Geografía [INEGI], 2017). This type of vegetation is among the most affected by human development and activities, being susceptible to forest fires, firewood extraction, and agricultural activities, especially in the center of the country (Challenger, 2003).
Ecological restoration is a relatively recent discipline in Mexico (Calva-Soto & Pavón, 2018). A viable strategy for restoration of degraded areas is reforestation with native species that, in the long term, favor the recovery of diversity and improve the conditions of the degraded site (Ventura-Ríos, Plascencia-Escalante, Hernández de la Rosa, Ángeles-Pérez, & Aldrete, 2017). For this purpose, it is convenient to select native species adapted to the conditions of the site to be restored, thereby achieving a higher percentage of success (González-Espinosa et al., 2007; Meli, Martínez-Ramos, & Rey-Benayas, 2013). A frequent obstacle is not having the native species to help meet the objective, due, in part, to the lack of biological information. It is essential to understand the methods of germination or vegetative reproduction (Bonfil & Trejo, 2010; Martínez-Pérez, Orozco-Segovia, & Martorell, 2006; Ramos-Palacios, Orozco-Segovia, Sánchez-Coronado, & Barradas, 2012) and to generate information that facilitates the propagation of native species with relevant characteristics for restoration. Seeds are the most common means for the reproduction of forest species under nursery conditions, so it is common to improve the proportion of seeds that germinate by pre-germination treatments (Comisión Nacional Forestal [CONAFOR], 2015; Martínez-Pérez et al., 2006). Another method is vegetative propagation, either by cuttings or layering. This can be a good option to produce species that are difficult to reproduce in their habitat or difficult to germinate under nursery conditions (Delgado, Cuba, Hechenleitner, & Thiers, 2008; Ramos-Palacios et al., 2012)
Arctostaphylos pungens Kunt is a common species in temperate vegetation such as oak, oak-pine, conifer, and temperate scrub forests. A. pungens is widely distributed from the southwestern United States to southern Mexico in states such as Oaxaca and Chiapas, at altitudes of 1 600 to 3 200 m (González-Elizondo & González-Elizondo, 2014; Martínez-Pérez et al., 2006; Rzedowski, 2006). This species has been mentioned as secondary vegetation and is considered a pioneer after disturbance by anthropogenic or natural causes (Díaz-Núñez, Sosa-Ramírez, & Pérez-Salicrup, 2016; Márquez-Linares et al., 2006; Sosa-Ramírez, Moreno-Rico, Sánchez-Martínez, Luna-Ruiz, & Siqueiros-Delgado, 2016), colonizing sites affected mainly by fires (Márquez-Linares et al., 2006). A. pungens is a shrub, rarely tree, 0.4 to 5 m in height, with exfoliating bark and reddish to reddish-purple color. Its leaves are leathery, elliptic and 1 to 3.3 cm long. The flower is white urceolate to Mexican pink and grouped in clusters of five to eight flowers. The fruit is a depressed globose edible drupe, smooth, 5 to 8 (11) mm, orange to dark red. The number of seeds varies from 4 to 7 (10), with hardened integuments, forming ossicles united in groups of two to three. Each seed is gore-shaped and averages 3.2 mm long and 2.6 mm wide (Márquez-Linares, Jurado, & González-Elizondo, 2006).
A. pungens has potential for restoration, either for soil retention capacity or for its role in soil formation, with good leaflitter production (Martínez-Pérez et al., 2006); in addition, it provides other benefits to the population through the production of edible fruits and wood for firewood, and medicinal uses (García-Regalado, 2014; González-Elizondo & González-Elizondo, 2014). As a pioneer species found naturally in disturbed sites, it probably has greater resistance to unfavorable conditions such as water stress and deteriorated soils (Meli et al., 2013). Under a good management regime, where fires and other disturbances are controlled, pioneer species help the establishment of species considered climax forests, as is the case of the genus Quercus (Márquez-Linares et al., 2006)
Despite its wide distribution and the fact that A. pungens is considered a species with relevant characteristics for restoration, there are not many studies on its propagation. Most of them come from manuals that provide little information on its propagation and from articles that address seed ecology or pre-germinative treatments that indicate low germination (Jurado, Márquez-Linares, & Flores, 2011; Martínez-Pérez et al., 2006). However, this does not mean these are the best possible methods, because other potential pre-germination treatments are not considered (Martínez-Pérez et al., 2006). There is no information of its propagation by alternative methods, such as reproduction by means of vegetative material.
Due to the above, the objective of the present study was to evaluate the germination of A. pungens seeds under pre-germination treatments, as well as vegetative propagation by cuttings and air layering to find the most appropriate method. The information generated may be useful in obtaining A. pungens plants for later use in the restoration of temperate forests.
Materials and Methods
Selection of biological material
Autumn is one of the seasons with presence of mature fruits in the area (Rubalcava-Castillo et al., 2020). On December 6, 2019, 100 mature fruits per specimen (n = 15) were collected in the temperate forest of the Área Natural Protegida (ANP) Sierra Fría, Aguascalientes (22° 11' 56.07 "N, 102° 37' 54.88" W, 2 670 m) (Figure 1). On April 21, 2020, plant material was collected to make cuttings, after twig growth. The collection was done with pruning shears at the "Rancho Piletas" property, inside the ANP Sierra Fría. The plant material was transported in plastic bags to conserve moisture.
Sexual propagation
Morphometric characterization of fruit and seed
A total of one hundred fruits were taken randomly from a composite sample of the total fruits collected. The fruits were weighed on an analytical balance and length and width were measured with a digital caliper (Surtek 122200, China); subsequently, the seeds were extracted manually. Free seeds extracted from each fruit (Figure 1D) were counted and 10 replicates of 100 seeds were weighed; also, length, width and thickness of 100 free seeds were measured.
Germination
Treatments consisted of trying to break the physical and physiological dormancy of the seeds. For this purpose, eight pre-germination treatments were tested to find the most effective method for propagation (Table 1). Sulfuric acid (H2SO4) was used at a concentration of 98 %. Stratification consisted of cold storage (4 °C). Heat shock consisted of placing the seeds in dry sand at 100 °C for 5 min, as a simulation of forest fires during the dry season (Zuloaga-Aguilar, Briones, & Orozco-Segovia, 2010).
Table 1 Pre-germination treatments on Arctostaphylos pungens seeds.
| Treatment | Treatment description |
|---|---|
| T1 | Control |
| T2 | Immersion in H2SO4 for 5 h |
| T3 | Immersion in H2SO4 for 6 h |
| T4 | Stratification for 90 days + heat shock |
| T5 | Stratification for 60 days + heat shock |
| T6 | Stratification for 30 days + heat shock + Stratification for 30 days + heat shock |
| T7 | Stratification 60 days + immersion in H2SO4 for 4 h |
| T8 | Immersion in H2SO4 for 5 h + immersion in gibberellic acid (GA3, 1 000 ppm) for 24 h |
H2SO4 was used at a concentration of 98 %, stratification consisted of cold storage (4 °C) and heat shock consisted of placing the seeds in dry sand at 100 °C for 5 min.
A total of four replicates of 30 free seeds per treatment were used in the germination tests (Jurado et al., 2011). Each experimental unit consisted of 95 x 15 mm Petri dishes, with kraft paper and cotton. Seeds were washed with 10 % chlorine for 5 min, then sown in the Petri dishes; a drop of Interguzan 30-30® fungicide was added to each seed to prevent fungal attack. Seeds were placed at 25 ± 2 °C with an 8-h photoperiod. Germination was recorded every two days for 60 days. At the end, the average germination percentage and mean germination time were calculated: TMG= Σ XiTi Σ Xi , where, Xi is the number of germinated seeds per observation and Ti are the days elapsed after sowing.
Asexual propagation
Cuttings
Cuttings consisted of recently grown (first 10 cm from the apex), semi-lignified shoots, 6 to 7 cm long, which the lower leaves were removed leaving only two or three near the apex (Delgado et al., 2008; Saldías, 2016). The cuttings were cut diagonally at the base to increase the absorption surface. Before placing the cuttings in the substrate, they were treated with a solution of Captan fungicide (1 g∙L-1) to prevent fungi; subsequently, the area of the basal cut was impregnated with the commercial product Radix® 10000 (powdered), which has indolbutyric acid (IBA) as active ingredient.
The experiment consisted of four replicates of 30 cuttings each, which were placed in hinge containers to conserve moisture, using previously moistened peat moss as substrate. The containers were kept in a greenhouse (15 °C minimum, 36 °C maximum and relative humidity 30 to 50 %) with light irrigation on Mondays and Fridays using an atomizer. In the first four irrigations, Captan was again applied directly to the base of the cuttings to prevent fungus formation.
Air layering
Air layering was carried out because natural vegetative propagation was observed in the field and has been mentioned as one of the main forms of natural reproduction in the collection region (Luna-Ruiz, Moreno-Rico, Sosa-Ramírez, & Sánchez-Martínez, 2016). Ten aerial layering per site were carried out on four populations of A. pungens at the Piletas Ranch, ANP Sierra Fría; each site represented one replicate. The shrubs had a diameter at the base greater than 15 cm and were 1.70 to 2.5 high.
Branches with a diameter of 1 to 2 cm and approximately 40 to 50 cm in length were used for air layering. A 2 to 3 cm ring of bark was removed from each branch. A rooting paste prepared with lanolin and Radix® 10000 was applied at a 4:1 ratio. Subsequently, a transparent bag (18 x 25 cm) was attached with string to place previously moistened peat moss as substrate, covering all the detached bark. The ends were tied to prevent moisture loss. The substrate was irrigated with 25 mL of water every five weeks using a 5 mL syringe.
Statistical analysis
Means and standard deviations were estimated for fruit characterization, seed weight, germination and vegetative propagation. The experimental design was completely randomized. The effect of pre-germination treatments was compared by one-way ANOVA with Tukey's comparison of means (P ≤ 0.05); percentage data were previously transformed with the Arc sen function. The TMG was estimated and compared (Tukey, P ≤ 0.05) between T2, T3, T7 and T8 which were the treatments with germination. T1 and T4 were excluded, because only one seed germinated in one replicate. Statistical analyses were carried out using the InfoStat program (Di-Rienzo et al., 2016).
Results and Discussion
Sexual propagation
The fruits collected measured less than 1 cm, weighed 0.19 ± 0.04 g and had three free seeds per fruit. Seeds were small with a weight of 1.22 ± 0.13 g per 100 seeds (Table 2).
Table 2 Morphometric values of fruit and free seeds of Arctostaphylos pungens.
| Fruit | Seed | ||||||
|---|---|---|---|---|---|---|---|
| Width (mm) | Length (mm) | Weight (g) | Free seeds per fruit | Length (mm) | Width (mm) | Thickness (mm) | Weight of 100 seeds (g) |
| 9.06 ± 1.22 | 6.65 ± 0.67 | 0.19 ± 0.04 | 3.43 ± 1.98 | 4.20 ± 0.53 | 2.96 ± 0.32 | 1.98 ± 0.30 | 1.22 ± 0.13 |
± standard deviation of the mean (fruits and seeds: n = 100; weight of 100 seeds: n = 10).
In Durango and in the Bajío and adjacent areas, the largest fruits have a diameter of 5 to 11 mm in diameter (González-Elizondo & González-Elizondo, 2014; Márquez-Linares et al., 2006). In this study, seed size was larger than that of Durango which measured 3.2 mm in length and 2.6 mm in width. Fruit and seed sizes vary according to the climatic and edaphological conditions of the site where they develop (Pozo-Gómez, Orantes-García, Rioja-Paradela, Moreno-Moreno, & Ferrera-Sarmiento, 2019). The compared areas may have similar climates, so the sizes are similar. The number of free seeds also coincides with the description made for those areas. Weight is a variable that is not considered in the botanical description, but it is important for nursery production, because it can be used to determine the number of seeds per kilogram and the amount of seeds to use (Apodaca-Martínez et al., 2019).
Germination results showed significant differences (P < 0.0001), ranging from 0 to 73.3 % (Table 3; Figure 2). The seeds with the highest germination percentage (66 to 73 %) were those subjected to T8, a scarification process with H2SO4 for 5 h + 1 000 ppm GA3, being similar to the T2 and T3 treatments exposed to the acid for 5 and 6 h. T7 (4 °C for 60 days + H2SO4 for 4 h) caused 27.5 % germination, and in the remaining treatments it was null or almost null (Table 3).
Table 3 Germination and mean germination time (MGT) of Arctostaphylos pungens seeds subjected to pre-germination treatments.
| Treatment | Germination (%) | MGT (days) |
|---|---|---|
| T1 (Control) | 0.83 ± 1.67 c | -18 |
| T2 (H2SO4 for 5 h) | 66.67 ± 9.81 a | 13.91 ± 1.10 ab |
| T3 (H2SO4 for 6 h) | 71.67 ± 15.75 a | 9.93 ± 1.47 a |
| T4 (4 °C for 90 days + heat shock) | 0.83 ± 1.67 c | -54 |
| T5 (4 °C for 60 days + heat shock) | 0 ± 0 c | - |
| T6 (4 °C for 30 days + heat shock + 4 °C for 30 days + heat shock) | 0 ± 0 c | - |
| T7 (4 °C for 60 days + H2SO4 for 4 h) | 27.50 ± 11.01 b | 22.02 ± 8.93 b |
| T8 (H2SO4 for 5 h + gibberellic acid [GA3, 1 000 ppm] for 24 h) | 73.33 ± 15.63 a | 11.55 ± 0.37 a |
Scarification was carried out with H2SO4 at a concentration of 98 %, stratification was based on cold storage (4 °C) and heat shock consisted of placing the seeds in dry sand at 100 °C for 5 min. Mean germination and GMT (± standard deviation; n = 120) with a letter in common are not significantly different between treatments according to Tukey's test (P > 0.05). Treatments T1 and T4 were excluded from the comparison of means, as only one seed germinated in one replicate; values in parentheses represent the day of seed germination.
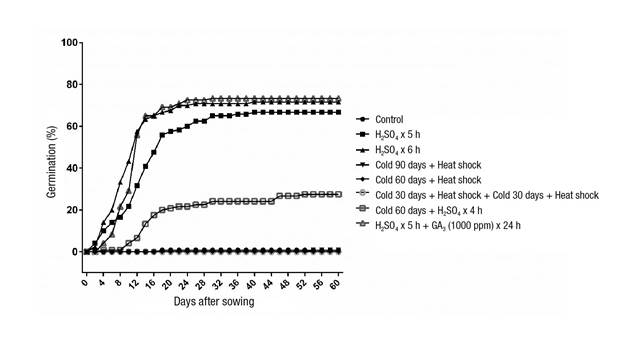
Figure 2 Cumulative germination curve of the eight pre-germination treatments in Arctostaphylos pungens. Scarification was carried out with H2SO4 at a concentration of 98 %, stratification was based on cold storage (4 °C) and heat shock consisted of placing the seeds in dry sand at 100 °C for 5 min.
H2SO4 is commonly used as a pre-germination treatment in species with impermeable covers that prevent the entry of water to the seed; this method has increased germination in species of the genera Medicago, Arctostaphylos and Juniperus (Martínez-Pérez et al., 2006; Peng, Xiao, Wang, & Yu, 2018; Tilki, 2007). Arctostaphylos pungens forms seed banks (Márquez-Linares et al., 2006), indicating that they are orthodox, and their testa prevents moisture ingress and egress, which leads to the need for testa degradation. Germination results for seeds treated with H2SO4 were higher than those reported by Martínez-Pérez et al. (2006), who reported 63 % germination with exposure to H2SO4 for 6 h and less than 10 % with 5 h of immersion. Moreover, these authors mentioned that germination was null with immersions for less than 5 h; in the present study, immersion for 4 h caused germination of 27.50 ± 11.01 %.
The percentages may differ due to the seed used in the experiments, because they are usually free or fused by two or more seeds (Jurado et al., 2011). This study used only free seeds; in the case of Martínez-Pérez et al. (2006) it is not mentioned whether free or fused seeds were used. In studies with Arctostaphylos uva-ursi (L.) Spreng. the difficulty of homogeneous germination is mentioned, due to the variety of seed size and the complexity of getting the time needed for H2SO4 to have the desired effects, in addition to the fact that small seeds can cause damage to the embryo (García-Fayos et al., 2001). In the case of A. pungens this may occur similarly, because the fused seeds could show a thicker testa, which would increase the immersion time necessary for H2SO4 to sufficiently degrade the mechanical barrier represented by the testa. It should be noted that differences in germination could also be due to variations among populations, origin, and seed production (Pozo-Gómez et al., 2019). According to the observations, it is recommended to choose free seeds of larger size to avoid possible damage caused by pre-germination treatment with H2SO4.
A. pungens, in combination with a very hard testa, it has a possible physiological dormancy (Jurado et al., 2011; Martínez-Pérez et al., 2006). The treatments that focused on breaking physiological dormancy, based on cold stratification together with heat shock, did not germinate except for one seed of T4 (4 °C for 90 days + 100 °C heat shock for 5 min). These data are lower than those found by Jurado et al. (2011), who focused on breaking dormancy from a point of view of A. pungens seed ecology and its relationship with fire, increasing germination by up to 29.7 ± 8.4 % with a combination of different factors (charcoal extract, smoke, cold, and heat). It should be noted that, in that study, germination percentages were relatively low with treatments similar to those used in this study, because with 40 days of cold storage plus thermal shock of 100 °C they achieved 5.7 ± 4.3 % germination.
The positive effect of cold stratification on germination has been mentioned in several species (Baskin & Baskin, 2004) and that of the combination with GA3 in species of the same family as A. pungens (Ericaceae) such as the genus Arbutus (Bertsouklis & Papafotiou, 2013; Smiris et al., 2006). Cold stimulates the breaking of physiological dormancy, as it happens in nature due to the effect of winter. Such dormancy can range from non-deep, intermediate, and deep; the latter requires three to four months of cold (Baskin & Baskin, 2004). This could indicate that A. pungens may have deep physiological dormancy and require more time in cold storage than that used in the present experiment (90 days).
In this study, treatments T7 (4 °C for 60 days + H2SO4 for 4 h) and T8 (H2SO4 for 5 h + 1 000 ppm GA3 for 24 h) sought to cover physiological and physical dormancy. For treatment T7, a longer cold storage period may have been necessary, as well as a longer immersion time in H2SO4. Treatment T8, with GA3 after the 5 h immersion in acid, although not statistically different, had a higher germination percentage and a lower GMT than the treatment that only consisted of immersion for 5 h in H2SO4. Other studies have mentioned the positive effects of GA3 on germination and dormancy breaking, replacing the need for a specific environmental stimulus such as temperature or light (Baskin & Baskin, 2004).
The time required for germination showed differences (P = 0.0128). GMT ranged from 9 to 22 days after sowing; treatments with H2SO4 had the shortest germination times, which started before 10 days after sowing, while the remaining treatments started on days 18 and 54 (Table 3; Figure 2).
The effect of H2SO4 was also reflected in the time required for the seed to germinate, being lower in seeds immersed for a longer time. H2SO4, by reducing the barrier represented by the testa of A. pungens, facilitates the entry of water into the embryo to initiate imbibition and, consequently, germination; moreover, it helps the radicle to break the testa (Martínez-Calderón, Sosa-Ramírez, Torres-González, Mendieta-Vázquez, & Sandoval-Ortega, 2020). Other studies mention a T50 (time needed to reach 50 % of total germination) of 18 days (Martínez-Pérez et al., 2006); the present study showed slightly shorter times of 16 days in the germination curve (Figure 2).
Asexual propagation
Cuttings showed 35.78 % survival at 12 weeks, but no root and callus were formed. The beginning of axillary bud development was observed after four weeks, but was not completed. This contrasts with that observed by Hart (2005) in other species of the genus Arctostaphylos with the possibility of rooting, differing in the use of the substrate (agrolite). Hart (2005) mentions that good aeration and drainage can be important factors for vegetative propagation and that, in medium-sized shrub or tree species such as Arctostaphylos grandulosa Eastw. cuttings can take three to five months to form roots. This may occur with A. pungens, being a large shrub species of 3 to 4 m, root formation would take longer.
Air layering showed no death of the branch used for layering, and there was callus (97.5 %) and root (37.50 %) formation. In general, after 20 weeks, the air layering had two to seven roots (80 %) newly formed; the rest had 10 to 18 roots (20 %). The size of these was mostly less than 10 mm (78.4 %) and the rest ranged from 10 to 25 mm (21.6 %).
Results show the possibility of inducing root formation for vegetative propagation of the species as observed in the wild (Luna-Ruiz et al., 2016) by air layering treated with AIB. This auxin has been successfully used to promote root formation in woody species useful for food, ornamental and, to a lesser extent, wild forest species (Abdel-Rahman, Abdul-Hafeez, & Saleh, 2020; Ramos-Palacios et al., 2012; Sánchez-Urdaneta et al., 2009).
The findings of the present study are similar to that occurred in a mangrove species (Conocarpus erectus L.) that formed shoots in cuttings but had no root development, while in air layering, root formation was achieved (Benítez-Pardo, Flores-Verdugo, & Flores-Verdugo, 2002). This may be because the species requires more than one season to achieve the formation of a good root system, which would not be achieved by scions or cuttings. Another factor that influences success is the season in which the vegetative propagation practice is carried out (Benítez-Pardo et al., 2002). In the present study, air layering was done in spring, reaching the end of their first growth stage, and they were removed in summer when they were in their second growing season.
As mentioned above, callus formation was observed in 97.50 ± 5 % of air layering. In various species, such a phenomenon may represent the onset of root formation as a consequence of cell differentiation (Ikeuchi, Sugimoto, & Iwasec, 2013). Callus formation is stimulated by the action of hormones (auxins and cytokinins), wounding, and genes involved in growth, developing a mass of undifferentiated cells and subsequent formation of plant organ regeneration, either shoot or root formation (Ikeuchi et al., 2013; Lozzi, Abdelwahd, Alami-Halimi, Mentag, & Abousalim, 2019; Lu, Liu, Lyu, Yuan, & Wu, 2019). Ikeuchi et al. (2013) mention that an intermediate ratio of auxins and cytokinins stimulates callus formation, while a higher ratio of auxins than cytokinins would stimulate root formation. This would indicate that A. pungens shows cytokinins that together with the auxins used (AIB) formed callus in most of the air layering, also accounting for the effect of the wound made in the air layering process. Callus formation in air layering, in other cases, can cover the cut and create a reconnection with the stock branch, delaying root formation (Sánchez-Urdaneta et al., 2009). This could also occur in the present study with A. pungens.
According to the results, both in cuttings and air layering, A. pungens is a species that requires more than one season for root formation. This makes it necessary to leave the air layering for more than 20 weeks to achieve root formation that allows the survival of the branch after separation from the mother bush, since air layering began to dry out a month after cutting because roots were not large enough to maintain the branch. There are no studies on vegetative propagation of A. pungens, which is common for wild forest species, although this form of reproduction can be an alternative for species with seed propagation problems (Benítez-Pardo et al., 2002; Ramos-Palacios et al., 2012).
The results contribute to the understanding of A. pungens and knowledge for the improvement of propagation in reforestation and restoration programs. Even so, more studies are needed to obtain the desired results, especially considering the variety of seed sizes and their behavior in each region, given its wide distribution in the country. The vegetative propagation of A. pungens should be further explored, being this study a first step and knowing that, in some areas, it is the main means of reproduction. It would be advisable to continue research on A. pungens under nursery conditions, considering emergence, survival, use of substrates and fertilization to complete an ideal propagation scheme.
Conclusions
Germination of Arctostaphylos pungens is improved by prolonged testa degradation in H2SO4. It is recommended to select larger seeds to avoid possible damage caused by the acid or to try other concentrations of H2SO4 to achieve testa degradation without having to discard small seeds, so that the genetic diversity of the species is preserved. Air layering can work as an alternative method to seed, leaving it for more than 20 weeks to achieve better root formation. It is recommended to continue experimenting with vegetative propagation to find the best method, trying different concentrations of indolbutyric acid, seasons of the year and substrates that allow greater aeration and drainage.











 texto en
texto en 



