Highlights:
Lupinus montanus produces over 5 500 seeds per plant in one reproductive cycle.
Seed production potential was positively associated with plant size.
The seed dispersal curve was adjusted to a negative exponential model.
Direction of soil slope had no impact on seed dispersal distance.
Most of the dispersed seeds (97 %) fell at a distance ≤2 m from the mother plant.
Introduction
Dispersal is defined as migration of an organism from its site of origin to the site where it is set up (van Den et al., 2016). Unlike mobile organisms, plant dispersal occurs when a seed, spore, or vegetative propagule detaches from its parent plant (van Den, La Rue, & Emery, 2016). This process links the reproductive cycle of plants with the establishment of their progeny (Wang & Smith, 2002). The spatial displacement of seeds is intended to increase the probability of establishment and colonization of species (Tercero-Burcado & Rovere, 2010).
The particular attributes of the seed dispersal process have important consequences for the evolution of ecological niches that determine where a species can persist (van Den et al., 2016). Dispersal facilitates the escape of seeds from predators (Perea, 2012) and decreases competition in areas close to the mother plant (van Den et al., 2016). A species with short dispersal distance or few dispersing propagules may not colonize suitable habitats beyond its current distribution limits, whereas widespread dispersal may increase the chances of colonization of habitats that at first glance seem inadequate (van Den et al., 2016).
The distribution of plant species, especially in mountain environments, has been modified by climate change and this has caused many of them to lag behind or have no possibility of adapting due to their strong local attachments (Jump & Peñuelas, 2005). Facing climate change, plants have options of adapting, migrating or dying (Aitken, Yeaman, Holliday, Wang, & Curtis-McLane, 2008). Adaptation possibilities depend on the level of genetic variation and phenotypic plasticity of populations to adjust their processes to new conditions (Soto-Correa, Lindig-Cisneros, & Sáenz-Romero 2014; Tognetti, Mazia, & Ibañez, 2019). On the other hand, the natural ability of plants to migrate through dispersal and set up in new habitats can be extremely slow for most species (Pearson, 2006; Soto-Correa et al., 2014).
In high mountain ecosystems, the main option for plant migration is to higher elevation sites, where temperature conditions to which they are adapted are expected to occur (Byars, Papst, & Hoffmann, 2007); however, the rate of altitudinal migration must be relatively rapid, before phenological changes limiting their reproductive capacity might occur, or being invaded and displaced by species from lower elevations (Peñuelas, Filella, & Comas, 2002). Climate change represents important challenges for forest managers and ecologists in terms of practicing effective management activities, whether for commercial or conservation purposes (Sáenz-Romero et al., 2016).
Lupinus montanus Kunth is a species of the Fabaceae family with a wide natural distribution in the mountains of Mexico (Dunn, 2001). Most of the natural populations of this plant are found in the Transmexican Volcanic Belt, at altitudes ranging from 2 500 to 4 100 m, associated with pine (Pinus hartwegii Lindl.), oak (Quercus spp.) and fir (Abies religiosa [Kunt.] Schltdl. & Cham.) forests, and alpine and subalpine grassland (Acosta-Percástegui & Rodríguez-Trejo, 2005), so it represents an important biological model to evaluate the altitudinal migration capacity under these conditions. Like other species of the same genus, L. montanus has a special seed dispersal mechanism (Abraham de Noir, Bravo, & Abdala, 2002). Pod-shaped fruits explode and throw seeds into the air in different directions (Ruiz, Gómez, & Lindig, 2010); that is, they have a ballistic dispersal. Dispersed seeds can germinate immediately or remain in the soil forming an ecologically and evolutionarily important seed bank in the population dynamics of many species (Ooi, 2012). Small seeds (i. e., <5 mm) as in the case of L. montanus, are easily buried and appear to be less at risk, although they may also be targets for earthworms, ants and other soil fauna (Thompson & Fenner, 1992). However, given the scenario of climate change, there is little information on the dispersal capacity of Lupinus and the presence of a natural seed bank that would allow us to visualize its long-term permanence or its possible migration to other areas within its alpine condition.
The objective of this study was to determine the seed bank of L. montanus, as a result of its dispersal in reproductive cycles of previous years; the relationship of plant size with seed production potential; and the distance of seed dispersal in the upper altitudinal limit of the natural distribution in the Nevado de Toluca. This information would help setting up the spatial pattern of dispersal and altitudinal migration potential of L. montanus.
Materials and methods
Study area
The study was conducted in the Natural Protected Area Nevado de Toluca (Área de Protección de Flora y Fauna Nevado de Toluca; APFF Nevado de Toluca, in Spanish), which includes the volcano with the same name (Figure 1). El Nevado de Toluca ranks fourth among the highest peaks in Mexico, with an elevation of 4 660 m and an area of 53 590 ha; it is located in the south-central portion of Estado de México, 23 km southwest of the city of Toluca (SEMARNAT, 2016). The predominant type of soil is Andosol, which occupies 90 % of the Natural Protected Area (INEGI, 2000). The climate is cold E(T)H wig and semi-cold-subhumid C(E)wig according to the Köppen classification modified by García (1973). Rainfall is in summer, although there may be snowfall in winter. Mean monthly temperature ranges between 2 °C and 5 °C, while extreme maximum temperature is 21 °C in summer and extreme minimum temperature is -10 °C in winter (SEMARNAT, 2016).
Identification of seed bank
The identification was carried out in August 2017, prior to the dispersal cycle of that year, with the purpose of counting seeds and considering them as a seed bank or reservoir of the species. Three sampling sites were selected and in each site four polygons of 25 m2 (5 m x 5 m) or subpopulations (patches with a minimum of 10 adult plants) located at the upper altitudinal limit of distribution of the species (4 200 m), separated by at least 100 m (Figure 1) were delimited. Polygons were divided into 25 squares of 1 m2 and five of them were randomly selected. In the center of each unit a soil sample was taken with a PVC cylinder of 20 cm diameter (314.16 cm2) and 5 cm depth. A total of 60 soil samples (1.88 m2) were collected and placed in plastic bags. Samples were dried at room temperature in a greenhouse and sieved with a 1 mm2 pore size mesh for separation and counting the number of L. montanus seeds.
Seed dispersal assessment
In October 2017, three subpopulations (patches of L. montanus) were selected at the upper altitudinal limit of natural distribution, near 4 200 m. Five plants in reproductive stage (15 plants in total) were located in each subpopulation, isolated from other adult plants. This number of plants was sampled because it was not possible to find a larger number of plants isolated from the population. Traps (plastic strainers) of 12 cm in diameter (113.09 cm2) and 5 cm high, having a plastic mesh inside to prevent seed bouncing when they fell inside, were placed at each individual plant. Traps were placed in the form of a cross with the plant in the center; from this point, 2 m-length lines were drawn in a parallel and perpendicular direction to soil slope. In the perpendicular direction, traps were placed every 50 cm from the center, and in the case of the parallel direction, they were placed every 25 cm; the main interest was to detect, with greater precision, the dispersion potential for and against soil slope. A total of 24 seed traps were placed around each plant.
The number of stems and pods was counted on each lupine, and height of the three tallest central stems was measured. Subsequently, to estimate production potential, the number of full seeds (with embryo) per pod was determined in a sample of 50 pods taken at random from 10 nearby plants. During the period from October 2017 to January 2018, the number of seeds collected in the traps was counted each week and the total number of seeds collected in each trap was obtained.
Statistical analysis
The number of seeds per m2 of soil was estimated with data from the seed bank. Seed production potential per plant was estimated with the average number of pods and seeds per pod. The relationship between seed production potential and plant size (height and number of stems) was established using Pearson's correlation. A one-way analysis of variance (P = 0.05) was also performed to evaluate differences in these plant characteristics among subpopulations.
The Kruskal-Wallis test was used to determine possible differences in the number of seeds collected between distances, for each of the orientations in which the traps were placed and in the average of all of them. Prior to comparisons between distances, the data on the number of seeds were transformed with the square root function.
Furthermore, with the number of seeds collected per trap, the dispersion curve was adjusted with respect to distance from the mother plant, using the Model procedure of SAS version 9.3 (Statistical Analysis System, 2013) to fit a negative exponential model (Bustamante, 1996) as follows:
where,
Y j = number of seeds collected in the j-th trap
X j = distance (cm) from the j-th trap to the mother plant
a and b = coefficients of the regression model to be estimated
The possible effect of orientation (for vs. against soil slope or parallel vs. perpendicular to soil slope) on the seed dispersal curve was determined based on the above model, from which the following equation was derived Y ij = (a + a oi )*exp [(b + boi)Xij] , where “oi” is the indicator variable for the i-th seed dispersal orientation.
Results and discussion
Seed bank in soil
A total of eight seeds of L. montanus were found in the sampled area (1.88 m2), which represents a density of 4.26 seeds∙m-2 of soil. All seeds were viable, because 100 % germination was obtained. Apparently, a significant amount of seeds remain stored in the soil for some time to form a persistent seed bank that can be short or long term, depending on climatic conditions of site and adaptive mechanisms of seeds (Marañón, 2001). Lupine seeds have physical dormancy and germination starts until favorable conditions appear, through some mechanism or stimulus (Pablo-Pérez, Lagunes-Espinoza, López-Upton, Ramos-Juárez, & Aranda-Ibáñez, 2013).
The main factors affecting seed persistence in the soil are predation and pathogen attack (De Souza-Maia, Maia, & Pérez, 2006); however, in arctic and alpine zones, incidence of these factors is low, which favors the maintenance of viable seeds in the soil (McGraw & Vavrek, 1989). Although L. montanus seeds can be consumed by insects, birds and rodents, the latter two groups of animals can also assist in dispersal, as has been shown for other species, including Fabaceae (Amico & Aizen, 2005; Pérez-Cadavid, Rojas-Soto, & Bonilla-Moheno, 2018); this process can amplify their altitudinal migration potential. The study of seed dynamics in soil is useful for understanding ecological processes involved in population migration (Pérez & Santiago, 2001).
Seed production capacity
A plant of L. montanus has the potential to produce over 5 500 seeds per reproductive cycle, with an average of 637 pods per plant (Table 1) and nine full seeds per pod. No significant differences (P = 0.43) were found among the sampled subpopulations in their productive capacity. Instead, significant differences were found with respect to the number of stems (P = 0.045) and plant height (P = 0.0004); moreover, the number of pods was positively correlated with the number (r = 0.8602, P < 0.0001) and height (r = 0.9539, P < 0.0001) of stems. In other words, seed production potential is strongly related to plant size, measured in terms of height or number of stems. Apparently, the reproductive strategy of the species is to produce as many stems as possible with the greatest possible length so that inflorescences are produced on each stem to maximize seed production. This is a general attribute of Fabaceae, where the production of resprouts or stems generating reproductive structures favors the permanence of adult individuals (Barchuk, Campos, Oviedo, & Díaz, 2006).
Table 1 Number of pods and seed production potential (± standard error) per plant (15 plants in total) in three subpopulations of Lupinus montanus in Nevado de Toluca mountain.
| Subpopulation | Pods per plant | Seed potential | |||
|---|---|---|---|---|---|
| Mean | Extreme values | Mean | Extreme values | ||
| 1 | 694.0 ± 50.8 | 433-1 016 | 6 246 ± 56.8 | 3 897-9 144 | |
| 2 | 595.2 ± 34.4 | 345-794 | 5 357 ± 309.5 | 3 105-7 176 | |
| 3 | 623.2 ± 12.9 | 521-699 | 5 608 ± 116.4 | 4 689-6 291 | |
The analysis of variance indicated no significant differences (P = 0.43) among sampled subpopulations.
The data show that this species has high seed production potential per plant and that they are maintained at shallow soil depths as is typical for Fabaceae (O'Connor & Pickett, 1992); however, in addition to seed consumption by fauna and pathogen attack, seedling establishment is a difficult and even uncommon process in high mountain environments (Cabrera, 2002). Therefore, high seed production can compensate for high seedling mortality due to adverse environmental factors such as freezing temperatures, soil instability, and nutrient shortages (Escudero et al., 2012).
Seed dispersal ability
The number of seeds collected varied significantly (P < 0.0001) in the traps among all distances from 25 cm to 100 cm. Figure 1 shows that the average seed dispersal curve with respect to distance was significantly (P < 0.01) fitted to a negative exponential model with R2 = 0.849. According to this model, 90 % of seeds dispersed by a plant fall within the first meter of distance and less than 3.2 % disperse naturally 2 m or more from the mother plant.
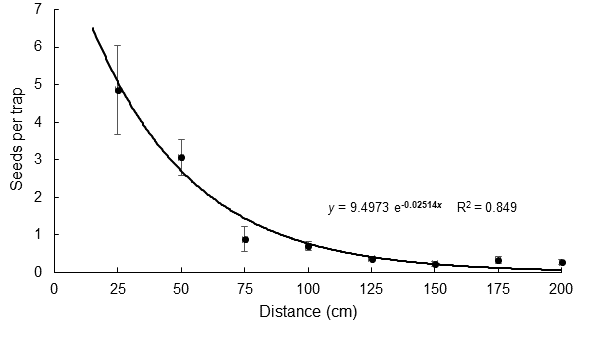
Figure 2 Spatial pattern of Lupinus montanus (15 plants total) seed dispersal averaged over the four trap orientations. The line indicates the distribution to which the data were fitted. Differences were significant (Kruskal-Wallis; P < 0.0001) among all distances from 25 cm to 100 cm.
These dispersal results agree with data presented by Ruiz et al. (2010) for Lupinus elegans Kunth, as they also found that the greatest number of seeds was concentrated near the mother plant, with a limited dispersal distance (i. e., <1 m). That study showed that more seeds were collected in traps placed downslope of plants, so that ascent to higher elevations was limited. However, geographic movement of seeds depends not only on dispersal from the mother plant; factors such as wind speed and direction, ingestion and transport by wildlife or rainfall events can cause seeds to move further away from the mother plant (Garcia, 1991), which may favor species migration to higher elevations.
This study showed no significant effect (P > 0.05) of orientation (parallel or perpendicular) when fitting the exponential model with indicator variables (“oi”). According to Table 2, estimated parameters for indicator variable when comparing orientation for vs. against soil slope or direction parallel vs. perpendicular to soil slope were not significant. Figure 2 shows that fitted seed dispersal curves are similar in both cases.
Table 2 Significance value (P) of parameters estimated in the negative exponential model fitted to the dispersal curve of Lupinus montanus (15 plants in total) seeds in different directions with respect to soil slope in the Nevado de Toluca mountain.
| Parameter | Significance | Estimated value | Standard error | P |
|---|---|---|---|---|
| For vs. against soil slope | ||||
| a | Base model (against soil slope) | 17.3063 | 6.4033 | 0.0130 |
| b | Base model (against soil slope) | -0.0487 | 0.0129 | 0.0011 |
| ao1 | Effect in favor of soil slope | -8.8809 | 7.3411 | 0.2328 |
| bo1 | Effect in favor of soil slope | 0.0249 | 0.0154 | 0.1117 |
| Parallel vs. perpendicular to soil slope | ||||
| a | Base model (parallel to soil slope) | 10.8482 | 2.0550 | <0.0001 |
| b | Base model (parallel to soil slope) | -0.0332 | 0.0055 | <0.0001 |
| ao1 | Effect perpendicular to soil slope | 5.9361 | 7.6688 | 0.4416 |
| bo1 | Effect perpendicular to soil slope | 0.0035 | 0.0098 | 0.7205 |
L. montanus seeds dispersed symmetrically in the four orientations. Soil slope (about 30 %) did not favor any specific orientation, for or against soil slope. Most Lupinus species have a ballistic (autochory) dispersal mechanism: pods dry and burst open, dropping seeds at most a few meters away from the mother plants (Nevado, Contreras-Ortiz, Hughes, & Filatov, 2018). Lupinus texensis Hook drops its seeds from 0 to 4 m (Turner, Huang, Cronk, & Rieseberg, 2017).

Figure 3 Spatial pattern of Lupinus montanus seed dispersal in Nevado de Toluca mountain: a) for and against soil slope; b) parallel and perpendicular to soil slope (15 plants in total). The lines show the curves fitted to each data series. No significant effect (P > 0.05) of orientation was found according to the regression model fitted for parameter estimation.
Seed dispersal is the most important demographic phase for plant migration across the land; seedling establishment and survival depend on adaptation, resistance and seed establishment mechanisms (Ooi, 2012). This study shows that, L. montanus releases a large number of seeds, which don’t reach a far distance from the mother plant, in each reproductive cycle. This proximity to the mother plant also has genetic consequences, because local establishment of propagules results in a clustering of genetically related individuals (Rovere & Premoli, 2005). However, if these patches persist, they reproduce and release seeds, which could represent an opportunity for the species to migrate to other locations and modify population and community dynamics of plants (Wang & Smith, 2002). With the increase in temperature associated with climate change, the process of species migration can cause displacements and a new distribution of species (Aitken et al., 2008). The evaluation of seed dispersal dynamics and demographic effects in species such as lupines contributes to the understanding of the possible impacts of climate change in high-mountain areas.
The study on seed dispersal contributes to the knowledge of colonization of new areas. It is important to continue with the long-term observation of seed dispersal of L. montanus because oscillation in time also marks a particular dynamic with respect to the elevational ascent or retreat of this species in the alpine zone. Sampling was carried out to a distance of 2 m from the mother plant, so that further studies could determine the maximum dispersal range by extending the distance of traps. According to the dispersal distance, there is a possibility for lupine to increase the colonization of sites at higher elevations; however, climate change occurring in a short period of time could be a determining factor in the establishment of lupine populations in those sites.
Conclusions
Lupinus montanus produces and disperses a large number of seeds, but most of these seeds remain close to the mother plant (≤2 m), which limits the speed of expansion to other areas. Nevertheless, the uniform distribution in all orientations could be a continuous advantage as the soil slope does not represent a limiting factor for the altitudinal migration of the species. The high reproductive potential of the species, positively associated with plant size, allows these seeds becoming incorporated into the soil seedbank, if they are not depredated, waiting for conditions that might allow their germination, or to take advantage of ingestion by animals to move to other areas and become established.











 texto en
texto en 



