Highlights:
- Regeneration of Polylepis australis in the montane forest of the Southern Andean Yungas is impacted by livestock.
- Livestock do not affect density of newly emerged P. australis (˂1 year) seedlings.
- Density of saplings (˃1 year and ˂30 cm) was three times higher in areas without livestock.
- Livestock decreased density of P. australis saplings from the first year of life.
Introduction
All along the Andes, from Venezuela and up to central Argentina, high altitude forests are dominated by the genus Polylepis (Rosaceae) (Segovia-Salcedo, Domic, Boza, & Kessler, 2018). Polylepis forests are unique ecosystems in the world that grow at high altitudes (between 1 800 and 5 000 m) and host several endemic species. In many places they form patches interspersed with high-altitude grasslands above the continuous forest line (>5 000 m).
Vegetation distribution is conditioned by the interaction between environmental and anthropogenic variables (Cingolani, Renison, Tecco, Gurvich, & Cabido, 2008; Díaz, Noy-Meir, & Cabido, 2001). There is worldwide debate as to whether the origin of the observed altitudinal limits is climatic or anthropogenic (Sarmiento, 2002). This debate was also introduced for Polylepis (Kessler, 2002) and studies point to strong human influences on the distribution and presence of mountain vegetation. One of the most frequent disturbances in these forests (Sarmiento, 2002) is extensive livestock, as it affects the structure (Homolka & Heroldová, 2003; Teich, Cingolani, Renison, Hensen, & Giorgis, 2005) and floristic composition (Hernández, Sánchez, Carmona, Pineda, & Cuevas, 2016) or maintains grasslands in potential forest sites (Rao, Iason, Hulbert, Elston, & Racey, 2003; Renison et al., 2015).
In South America, Polylepis mountain forests are endangered due to logging, fire, and grazing caused by extensive livestock (Renison, Morales, Cuyckens, Sevillano, & Cabrera, 2018; Zimmermann, Renison, Leyer, & Hensen, 2009). However, livestock is the main economic activity in many mountain ecosystems, such as in the study site, where other labor is complex to perform due to the relief of the site. Understanding these systems is important for sustainable livestock management (Garin, Aldezabal, Herrero, & García-Serrano, 2000); therefore, their effect on high altitude forests must be better understood.
Extensive livestock impacts can be direct and indirect. Livestock affect directly by browsing and trampling (Dezzotti, Sbrancia, Rodríguez-Arias, Roat, & Parisi, 2003; Mountford, 2003; Teich et al., 2005; Torres, Renison, Hensen, Suarez, & Enrico, 2008) and indirectly by fires caused by farmers to promote pasture production. These practices cause forest fragmentation and reduction of tree size (Hensen, 2002; Renison, Hensen, Suarez, & Cingolani, 2006).
The stages of a tree are affected differently. Newly emerged seedlings have different defense mechanisms as adult individuals against herbivory (Fenner & Thompson, 2005). In this sense, livestock can prevent seed germination and kill newly emerged seedlings (<1 year old) by trampling, while browsing affects saplings (Dezzotti et al., 2003; Mountford, 2003) and reduces the reproductive ability of adults, due to the excessive extraction of biomass (Cierjacks & Hensen, 2004). However, the most vulnerable stages are the early stages: germination, seedling emergence and the first year of life (Bricker, Pearson, & Maron, 2010; Harmer, 2001; Kauffman & Maron, 2006).
In a livestock exclusion experiment in cloud forests of northwestern Argentina, saplings had greater height and density (Mazzini, Relva, & Malizia, 2018). In the Sierras Grandes de Córdoba (central Argentina), cattle grazing, together with fire associated with livestock management (fires to stimulate grazing saplings), is a key estimator of vegetation structure in P. australis forests (Cingolani et al., 2008; Renison et al., 2006; Teich et al., 2005; Torres et al., 2008) and influences its altitudinal distribution (Argibay & Renison, 2018). At the distribution limit of this species (northern Argentina), Renison et al. (2013) found some of the least conserved forests, so further studies in this sector are needed.
In this study, a closure experiment was conducted in a northern sector in the distribution of P. australis with the objective of estimating the effect of livestock (presence and absence) in the early stages of regeneration and to know how seedling and sapling densities vary over a year. It is assumed that the presence of livestock negatively affects natural early regeneration, through decrease of seedling and sapling densities of P. australis.
Materials and methods
Study species
The genus Polylepis is endemic to the mountains of South America where canopy of montane forests and shrublands predominate (Renison et al., 2013). Polylepis australis, a species of the Rosaceae family, is distributed only in Argentina in the Yungas ecoregion, along the eastern slopes of the Andean Mountains in the north of the country and in the Chaco serrano in the Sierras Grandes in the center. The species occupies the southernmost portion of the genus (hence the name). In the Yungas of Jujuy, P. australis grows at altitudes of 2 500 to 3 400 m in the ecotone, between high altitude grasslands and montane forest (Renison et al., 2013), where it forms monospecific forests, although it is sometimes accompanied by the Andean alder (Alnus acuminata Kunth). Polylepis forests are involved in the regulation of water resources, carbon sequestration, soil fixation and provide habitat for numerous species, including endemics species (Cuyckens & Renison, 2018). Polylepis forests are a fundamental source of wood as fuel for cooking food, making handicrafts, and making poles, beams and tools (Cuyckens & Renison, 2018). Trunk layers have a medicinal use: a coffee-colored infusion is made that frees the respiratory tract and cures kidney diseases; it is also used to dye fabrics (Neto, Vaisberg, Zhou, Kingston, & Hammond, 2000). The leaves are small, compound, imparipinnate, with serrated edge and arranged in fascicles on brachyblasts; they have an average of seven leaflets, but the first true leaves (nomophyllous) have three. The flowers are hermaphrodite, inconspicuous and arranged in racemes. The fruits are achenes (Fjeldså & Kessler, 2004).
Study area
The study was conducted in the montane forest of the northern sector of the Southern Andean Yungas. The ecoregion of the Southern Andean Yungas or Tucuman-Bolivian rainforest extends along the Andean Mountains from Bolivia (Tarija) to the province of La Rioja in Argentina, divided latitudinally into northern, central and southern sectors, separated by xerophytic forests (Burkart, Bárbaro, Sánchez, & Gómez, 1999). At an altitudinal level, the Yungas develop approximately between 400 m and 2 300 m, where three altitudinal levels can be recognized: pedemontane forest (400 to 700 m), montane rainforest (700 to 1 500-1 700 m) and montane forest (1 500-1 700 to 2 300 m); after these are the high-altitude grasslands (Grau & Brown, 1993). Montane forests are characterized by being less rich in tree species than rainforests; the most common are Prunus tucumanensis Lillo (palo de luz), Podocarpus parlatorei Pilg. (pino del cerro), A. acuminata, Sambucus peruviana Kunth (saúco) and Solanum trichoneuron Lillo (Cuyckens, Malizia, & Blundo, 2016). The climate of the Southern Andean Yungas is temperate and humid with mainly summer rains (80 %) and frosts during the winter (Bianchi, Yañez, & Acuña, 2005).
The Parque Provincial Potrero de Yala (PPPY) is located in the northern sector of the Southern Andean Yungas Australes (24° 05′ 29.16″ S, 65° 30′ 15.36” W, 2 619 m; Figure 1) and extends altitudinally between 1 600 and 5 000 m, using 4 300 ha. The PPPY is the core zone of the Yungas Biosphere Reserve and, therefore, the presence of exotic livestock is contradictory to its creation objectives; however, extensive livestock practices predate the park's creation, so it would be important to have management guidelines to ensure their coexistence. The PPPY as its name indicates was historically used for extensive livestock and according to Osuna and Guzmán (2014) it has, on average, a livestock density of 0.23 head of cattle per hectare; goats and sheep are also present in the study site. The area shows signs of overgrazing such as the abundance of Senecio rudbeckiaefolius Meyen et Walp. and the presence of grass for the sheep Alchemilla pinnata Pilg. ex Rothm. (Braun-Wilke et al., 2013). In PPPY, P. australis is found in pure forest or accompanied by A. acuminata, S. peruviana, Schinus gracilipes I. M. Johnst., Juglans australis Griseb. (nogal) and P. parlatorei.
Research on the site
Three exclosures of approximately 35 m2 were established in a monospecific P. australis forest, excluding large and small livestock. Over the course of one year (April 2014 to February 2015), seedlings and saplings were monitored in the exclosures and in a control area (with cattle [0.23 head∙ha-1] and small livestock [goats and sheep at unknown density]) outside the exclosures that had no observable environmental differences. The number of seedlings and saplings were quantified in three randomly arranged quadrats (1 m x 1 m) inside the exclosure and in three random quadrats in the designated area outside the exclosure. Data were recorded six times throughout the year for seedlings and four times for saplings. A seedling is defined as a recently emerged individual (i.e., less than one year old, with cotyledons and tiny size [˂1 cm]). A sapling defined as an individual that has lost cotyledons and measures less than 1 cm in diameter at the base (DAB). It is important to establish the criteria for DAB, because old Polylepis plants can be kept small by constant browsing. Small seedlings are recognizable by the presence of nomophylls typical of the genus (Figure 2).
Statistical analysis
Averages of seedling and sapling density were calculated at each measurement time and in each situation (presence vs. absence of livestock); averages were plotted over the years. Linear mixed models (normal distribution) were adjusted for seedling and saplings density for statistical analysis. The explanatory variables were livestock (presence vs. absence) and time of measurement (six months for seedlings and four months for saplings), and interaction between both variables was considered. Normality of residuals and and absence of overdispersion were analyzed for both models. The difference between treatments was verified by a Chi-square test (χ2) for seedling densities and for the densities of saplings. In all cases, the minimum adequate model was identified using the hypothesis testing method. Analyses were performed using MASS (Venables & Ripley, 2002) and ggplot2 (Wickham, 2016) packages of R software (R Core Team, 2020).
Results
Table 1 shows the results of the P. australis seedling density model depending on livestock presence and time of measurement. Throughout the year, mean seedling density with standard deviation was 9.06 ± 18.01 individuals∙m-2 in the absence of livestock and 4.50 ± 3.82 individuals∙m-2 in the presence of livestock; however, this difference was not statistically significant (P = 0.2). Therefore, according to the model, the presence of livestock did not affect seedling density. In contrast, according to the model, there are significant differences in seedling density according to the time of year (P < 0.001); this is because the difference between observations was large in the first measurement (April); however, it was reduced in the following months (both lines overlap), as shown in Figure 3. Interaction between the variables time and livestock was not significant, indicating that only time of measurement affected seedling density.
Table 1 Model of seedling density depending on livestock (presence vs. absence) and time of measurement over a one-year period in a queñoa (Polylepis australis) forest at Parque Provincial Potrero de Yala (Jujuy, Argentina).
| Explanatory variables | χ2 | gl | P |
|---|---|---|---|
| Momentum*livestock | 7.24 | 5 | 0.20 |
| Livestock | 1.04 | 1 | 0.31 |
| Momentum | 32.92 | 5 | 0.000004* |

Figure 3 Seedling density in presence and absence of livestock over the years in a queñoa (Polylepis australis) forest at Parque Provincial Potrero de Yala (Jujuy, Argentina).
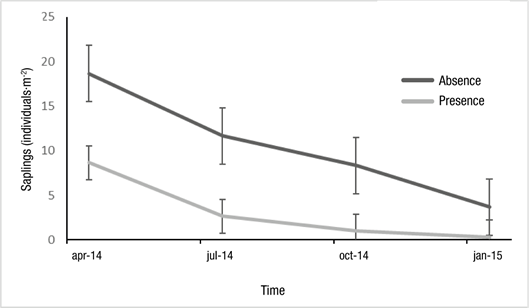
Table 2 shows that livestock presence significantly affected (P < 0.0001) sapling density; throughout the year, in areas without livestock (10.58 ± 6.64 individuals∙m-2) it was three times higher than with presence (3.17 ± 3.86 individuals∙m-2). Interaction between time of measurement and livestock was significant (P = 0.04); in both situations (with and without livestock), the density of saplings was higher at the first measuring point (Figure 4).
Table 2 Model sapling density depending on livestock (presence vs. absence) and time of measurement over a one-year period, in a queñoa (Polylepis australis) forest at Parque Provincial Potrero de Yala (Jujuy, Argentina).
| Explanatory variables | χ2 | gl | P |
|---|---|---|---|
| Momentum*Livestock | 8.045 | 3 | 0.04* |
| Livestock | 50.64 | 1 | ˂0.0001** |
| Momentum | 64.90 | 3 | ˂0.0001** |
Discussion
Presence of livestock had no effect on the density of P. australis seedlings in this sector of distribution, taking into account annual averages. This indicates that there are still seed trees and there is no limiting factor for natural regeneration, in contrast to that found by Torres et al. (2008) found for the same species in the Sierras de Córdoba; however, during the first month of measurement (April 2014) there was a difference in germination success between situations with and without livestock. If data had been taken only in April, differences would be significant and would lead to wrong conclusions; therefore, it is very important to make several measurements over time.
In more southern regions, livestock have been shown to promote soil compaction by trampling, which prevents germination of P. australis (Renison, Hensen, & Cingolani, 2004) and kills newly germinated seedlings (Dezzotti et al., 2003; Mountford, 2003). However, in the present study, the decrease in seedling density after April (Figure 3) occurs both in the presence and absence of livestock indicating that causes of mortality are unrelated and possibly due to environmental and biological factors (e.g., water scarcity and competition). In this regard, the study site has a marked dry season (October-November) and 80 % of rainfall occurs in summer (December-March) (Bianchi et al., 2005). It is likely that the peak of fruit ripening in P. australis occurs at the end of the dry season, as occurs in Polylepis tomentella Weddel (Domic, Mamani, & Camilo, 2013; Reboratti, 2006), and that a peak in germination is naturally generated at the end of the wet season when soil is completely saturated and coincides with the month of April. After the wet season, during the dry period, there would be no more seedling emergence, but there would be a natural drought mortality. In other words, the decrease in density after April indicates, on the one hand, that no more seeds germinate during the following months and, on the other hand, that the seedlings germinated in that month do not survive the first year. Seedlings may die after a few days due to lack of water (Reboratti, 2006) and the newly emerged seedlings often do not have the capacity to resist adverse conditions (water or thermal stress) tolerated by adult plants (Fenner & Thompson, 2005). Another factor could be the herbaceous stratum that is generated after the wet season and that negatively affects Polylepis seedlings due to competition for resources (George & Bazzaz, 1999). The herbaceous stratum at the study site is composed of numerous native and exotic feral plants (Festuca sp. and Duchesnea indica [Andr.] Focke) and herbivory-tolerant species (Digitalis purpurea L. and Alchemilla pinnata [Ruiz & Pav.] Rothm).
The next stage in the regeneration process are saplings that are more than one year old. This stage was affected by livestock; 3.3 times more saplings were found in the absence of livestock. This value is similar to that found by Torres et al. (2008) in the Sierras de Córdoba, who demonstrated that sites with low livestock density have between 3.5 and 4 times more P. australis saplings (individuals smaller than 5 cm) than in degraded sites. At PPPY, livestock grazing was observed on all visits and at all times of the year; in addition, due to the practice of transhumance, pressure could increase considerably in summer. As trees grow, the direct effect of livestock would change from trampling to browsing; for example, specimens of live, but browsed, saplings could be observed (Figure 5). It would be interesting to carry out follow-up studies of saplings by measuring their heights; however, in both situations (with and without livestock), there is a decreasing trend in the number of seedlings and saplings, so P. australis seems to have a high natural mortality rate, at least during the first years of life.

Figure 5 Saplings of Polylepis australis with evidence of browsing by livestock at Parque Provincial Potrero de Yala, Jujuy, Argentina.
At PPPY, the removal of leaflitter that facilitates germination is still carried out by large native herbivores. Red brocket deers (Mazama sp.) and collared peccary (Pecari tajacu L.) are present in the area (Malizia, Bergesio, Reid Rata, Cáceres, & Fierro, 2011). In the Sierras Grandes de Córdoba, Teich et al. (2005) and Zimmermann et al. (2009) found that both excessive livestock density and total exclusion negatively affect P. australis forest regeneration, while moderate grazing facilitates seed germination due to leaflitter removal. These studies were conducted at sites where native large herbivores were extinct and exotic livestock seem to have replaced their ecological function.
To promote Polylepis forest restoration, it would be important not to sow seeds, since these, despite germinating with relative success in the absence of cattle, would not survive the second year of life. In areas with presence of livestock it is necessary to determine an access where livestock is not an obstacle for natural regeneration; for example, Giorgis, Cingolani, Teich, and Poca (2020) recommended a density of livestock below 0.12 units∙ha-1 for the Sierras Grandes de Córdoba. This would mean that in PPPY livestock would have to be reduced by half; however, this must be defined locally, since the Yungas is an ecoregion with more abundant rainfall and probably a greater density capacity; in addition, it is necessary to know the abundance of native herbivores. Especially, the PPPY is a core zone of the Yungas biosphere and as a park it has a legal framework for protection, and it is possible to restore Polylepis forests (specific and the area in general). There are still seed trees and, therefore, there is potential for natural regeneration.
This study presents preliminary data that support the hypothesis that livestock affect saplings older than one year, but not in the first year. To understand the natural regeneration of the forest, the study should be extended to other stages of the life cycle; for example, Giorgis et al. (2020) found that livestock density that allows horizontal growth in adult trees is even lower than that which allows growth in height. Other factors that could be affecting P. australis forests are fire and the invasion of exotic species (Renison et al., 2013). Exotic herbaceous species (D. purpurea and D. indica) and pine plantations (Pinus patula Schiede ex Schltdl. & Cham. and Pinus taeda L.) are abundant in the PPPY.
The results of this study show anthropogenic influence on the early stages of natural regeneration in a Polylepis forest. Although seed trees still exist, the differences currently found will affect the structure of the forest in the long term; even if recovery is allowed, certain diameter classes will be absent. Studies of structure in P. australis forests in the Sierras Grandes de Córdoba (Teich et al., 2005) also indicate important anthropogenic impacts.
It would be important to extend the study through exclosures to other elevations, where the effect of the same number of livestock could be different, since this research was conducted at a single altitudinal level and was based on a small number of exclosures, therefore, the results are not definitive. Currently, some authors of the present study are developing a study involving more closures and the entire local gradient. The results of the present study are the first results of an experiment in this sector of the distribution of P. australis and are the initial step to contribute to the conservation of the species in the northern part of its distribution.
Conclusions
Natural regeneration of Polylepis forests would be affected if livestock activity is maintained under current management conditions, especially after the first year of life (saplings). Polylepis forests show no impact on germination, indicating that they still have the potential for natural regeneration. This indicates that urgent livestock control and management measures are needed over the entire distribution range. Differences found over time point to the need for long-term monitoring of seedlings. Differences found with research further south in the species' distribution highlight the importance of local studies.











 texto en
texto en