Introduction
Pinus radiata D. Don has great global economic importance, as it is one of the most planted pines (4.1 million ha approximately), mainly in the southern hemisphere (New Zealand, Chile, Australia, Argentina and South Africa) (Rogers, Matheson, Vargas-Hernández, & Guerra-Santos, 2006). The species is located in a reduced geographical area, with a climate influenced by the cold currents of the Pacific Ocean (McDonald & Laacke, 1990). Pinus radiata has five native populations; three are on the west coast of California (Hoeksema, Vargas-Hernandez, Rogers, Luna-Mendoza, & Thompson, 2012) and the other two are on the Mexican islands of Guadalupe and Cedros in the state of Baja California (Rogers, 2002). These two populations have a Mediterranean climate, with extreme temperatures due to their altitude of over 290 m (Rogers, Vargas-Hernandez, Matheson, & Guerra, 2005), although their origin is drastically different; Cedros Island has a continental origin, geologically similar to the Sierra Vizcaino (Rogers et al., 2005), and Guadalupe Island is part of an archipelago of volcanic origin, formed seven million years ago, which has never had contact with the continent (Ledig, Vargas-Hernández, & Johnsen, 1998). This causes populations to differ from each other in several adaptive traits (Millar, 1999) such as growth rate and salinity tolerance (Burdon, 2001), but their level of cold tolerance is unknown (Mead, 2013).
Low temperatures and drought represent the most important environmental constraints on plant distribution (Harrison et al., 2010) and productivity (Anjum et al., 2011). Both environmental variables change with altitude (Viveros-Viveros et al., 2009), latitude, longitude (Robson, Rasztovits, Aphalo, Alia, & Aranda, 2013) and with time of year (Charra-Vaskou, Charrier, Wortemann, & Beikircher, 2012). The negative effects that these factors have on the growth, productivity, and survival of individuals promote evolutionary adaptation and population differentiation (Blödner, Skroppa, Johnsen, & Polle, 2005; Larcher, 2005; Palacio, Milla, & Montserrat-Martí, 2005).
In P. radiata, early (autumn) and late (spring) frosts damage the meristematic tissues and juvenile foliage of seedlings (Greer, Robinson, Hall, Klages, & Donnison, 1998). In these periods, resistance to low temperatures varies widely, due to phenological differences in shoot growth (Neuner, 2014). Bachofen, Wohlgemuth, Ghazoul, and Moser (2016) found that cold resistance did not differ among populations of P. sylvestris L., P. nigra Arnold, and P. halepensis Mill., but did decline from winter to spring (February to May); in addition, drought-adapted populations of P. sylvestris and P. nigra were frost-resistant during the most sensitive phase (spring).
Despite the temperature increase expected in the coming decades (Intergovernmental Panel on Climate Change [IPCC], 2013), climate models reveal the persistence of irregular cold extremes in winter (Kodra, Steinhaeuser, & Ganguly, 2011). These abrupt changes in temperature often exceed the threshold of tolerance, inducing short- and long-term damage (Man, Lu, & Dang, 2017a; Williams, Henry, & Sinclair, 2015). Plants are more prone to freeze damage with these unexpected fluctuations (Kalcsits, Silim, & Tanino, 2009; Matusick, Ruthrof, Brouwers, & Hardy, 2014), due to phenological asynchrony with the environment (Ensminger, Hüner, & Busch, 2009; Man, Lu, & Dang, 2017b). Unusual fluctuations in temperature and humidity can generate severe stress conditions, to which natural populations are not adapted, causing negative effects on growth and survival. In this sense, the objective of the present study was to determine the differences in growth and tolerance to low temperatures in primary leaves of P. radiata seedlings from Cedros and Guadalupe islands, growing in two environments (greenhouse and outdoor) and different humidity conditions.
Materials and methods
Germplasm selection
P. radiata germplasm from two native populations on Cedros Island and Guadalupe Island, both in the state of Baja California, Mexico, was used. Two subpopulations were included from each population, spatially separated by latitude and altitude (Table 1).
The seed was sown in 200 mL containers, in a substrate of bark, perlite and tepezil (50:30:10) and 5 g·L-1 of controlled-release fertilizer (Multicote®) with irrigation at field capacity. After six months, seedlings of homogeneous size were selected from each subpopulation for the experiment: 29.8 ± 2.3 cm in height for seedlings from Guadalupe population, and 22.4 ± 2.1 cm in height from Cedros.
Table 1 Geographical location of Pinus radiata seed sources and mean annual temperature (MAT) and precipitation (MAP) conditions on the islands.
| Population | MAT1 (°C) | MAP1 (mm) | Subpopulation | Latitude (N) | Length (O) | Altitude (m) |
|---|---|---|---|---|---|---|
| Cedros Island (C) | 19.9 | 85 | C1 | 28° 10.741' | 115° 12.570' | 519 |
| C2 | 28° 15.314 | 115° 12.883' | 496 | |||
| Guadalupe Island (G) | 17.7 | 130 | G1 | 29° 09.357' | 118° 18.387' | 988 |
| G2 | 29° 09.600' | 118° 17.527' | 710 |
Establishment of the experiment
The experiment was set up in the nursery of the Posgrado en Ciencias Forestales from Colegio de Postgraduados, Montecillo campus, in two growth environments: individual containers (greenhouse) and growth beds (outdoor). During the period December 2018 to March 2019, the average temperature was 4.7 °C higher and with less diurnal fluctuation in the greenhouse (14.8 °C with average minimum of 6.3 °C and average maximum of 24.4 °C) than outdoors (10.1 °C with average minimum of 0.4 °C and average maximum of 22.8 °C). The average relative humidity in the greenhouse was 63.3 % with an average diurnal variation of 28.8 to 98.3 %, and outdoors it was 57.3 % with an average variation of 24.6 to 93.1 %. In the greenhouse, on October 26, 2018, 96 seedlings from each subpopulation were transplanted into 10 cm diameter and 100 cm long PVC pipes containing a substrate of local agricultural soil, sand and tepezil in a 50:30:20 ratio. Outdoors, on 22 November 2018, 80 seedlings from each subpopulation were transplanted at 20 cm x 15 cm between rows and seedlings in growth beds with local agricultural soil. Two moisture treatments (S0 and S1) were defined in each environment based on the substrate retention curve. At the S0 level, the moisture content was maintained between 76 and 100 % of its usable moisture (40 to 47 % moisture in containers and 26 to 30 % moisture in growth beds), while at the S1 level, the moisture content in containers was maintained between 10 and 25 % of the usable moisture, and without irrigation in the growth beds, preventing the entry of water during the rainy season. In each environment a 2 x 4 factorial split-plot design was used. In the large plots the moisture treatments (S0 and S1) were established, and in the small ones, the subpopulations with four replications. Each experimental unit was represented by 12 seedlings in the greenhouse and 10 seedlings in the growth beds.
Assessment of temperature damage
Based on preliminary tests (January 2019) to determine leaf type and stem position, it was decided to use three primary needles close to the apex per seedling in a sample of six seedlings randomly chosen at per experimental unit. The same seedlings were used in all tests. The needles were washed with distilled water and cut into 10-mm sections, discarding the tip and base. Each sample was separated into two parts with similar fresh weight, measured on an analytical balance (Chyo Balance Corp, precision 0.1 mg), and placed in test tubes; one part was subjected to freezing (Ft) and the other was used as reference or control (Fc).
The first cold-exposure test was done during the winter (6 February 2019) and the second was done in the spring (24 April 2019). On each occasion, the tubes with the Ft fraction were placed in racks inside a freezing chamber model M1212 (Manufacturas Ind. Universo, Mexico, 2013), with automatic temperature control, and the tubes with the Fc fraction were kept at room temperature (22 to 23 °C). The Ft fractions were subjected to a routine of gradual temperature decrease in the chamber, starting with a stabilization at 4 °C for one hour and then a decrease of 2 °C per hour, during 8 h, until reaching -12 °C. The samples were kept at this temperature for 4 h and then allowed to rest until they reached 4 °C again, to start the evaluation.
The damage caused to the needles was determined with the electrical conductivity, which measures the concentration of electrolytes released by the seedling tissues after exposure to freezing temperatures (Climent, Costa e Silva, Chambel, Pardos, & Almeida, 2009). After subjecting the Ft fraction to freezing, 10 mL of deionized water was added to the two fractions (Ft and Fc) and left to stand for 24 h at room temperature. The electrical conductivity (C1) was measured with a LAQUAtwin conductivity meter model EC-33. The samples were then sterilized in an autoclave at 120 °C for 15 minutes to kill the tissue and measure the total electrical conductivity (C2) 12 hours later. Three conductivity readings were taken for each sample every 20 minutes and the average of these was used in the damage calculations. With the electrical conductivity data standardized to a mass of 100 mg of tissue, the relative conductivity (R = 100*C1/C2) of each fraction (Rt and Rc) was calculated. The cell damage index (CDI) for each sample was calculated as CDI = 100 * (Rt - Rc)/(100 - Rc).
Evaluation of terminal shoot growth
The absolute growth rate (AGR, mm·d-1) of the terminal shoot was obtained by measuring the total height of the seedlings every two weeks, from 26 October in the greenhouse and from 22 November in the growth beds (outdoors), up to 8 May under both conditions. The AGR for each seedling (Arias et al., 2019) was calculated with the formula AGR = (A2 - A1)/(T2 - T1), where A1 and A2 are the initial and final seedling height (mm), respectively, at the initial (T1) and final (T2) time of each measurement period.
Statistical analysis
The data were subjected to an analysis of variance with the MIXED procedure in SAS 9.0 (SAS Institute, 2003), to assess the effects of soil moisture treatment and of populations and subpopulations in each environment separately, using the statistical model:
where,
Yijkl |
value of the ijkl-th observation |
µ |
overall average |
Bi |
block effect |
Sj |
effect of moisture treatment |
Bi*Sj |
effect of interaction block x moisture treatment |
Pk |
population effect |
Sj*Pk |
effect of interaction moisture treatment x population |
Subl(Pk) |
effect of subpopulation within population |
Sj*Subl(Pk) |
effect of interaction moisture treatment x subpopulation within population |
Eiikl |
experimental error |
Pearson's correlation between DI and AGR was estimated with the average DI values per subpopulation in each moisture treatment (n = 8) and period for each environment, and the average AGR data during the measurement periods. In addition, for each moisture treatment (n = 16), a correlation analysis between the DI and the AGR was performed with the average values per subpopulation, including the two growth environments and the two evaluation periods.
Results and discussion
Low-temperature damage
In winter an average DI of 50 % was obtained, while in spring it was 64 %. The DI caused by low temperatures in the tissues was similar both in favorable humidity and in drought conditions; however, significant differences (P < 0.05) between populations were found, except in spring for the seedlings growing outdoors. At subpopulation level, only outdoor seedlings showed significant differences (P < 0.01) during winter. In the same way, the interaction of humidity treatment*population was significant only in spring inside the greenhouse (P = 0.02).
Under natural conditions, P. radiata populations are exposed to extreme minimum temperatures of -5 °C with 300 frost-free days per year (McDonald & Laacke, 1990); however, in studies conducted outside their natural environment, the foliage of seedlings under two years of age suffers deterioration when exposed to temperatures between -3 and -6 °C in summer and between -12 and -14 °C in winter (Burdon, 2001). In this study, DI in primary needles of 10 and 12-month-old seedlings was 50 and 64 %, during winter and spring, respectively, with a lethal temperature (TL50) close to -12 °C. Climent et al. (2009) report a TL50 between -9 and -12 °C in 18-month-old seedlings of the same species.
Differences between populations and subpopulations
Figure 1 shows the DI in the assessed P. radiata populations and subpopulations. In the greenhouse, the Guadalupe island population presented higher DI in winter (59.9 %) and spring (75.0 %) than Cedros island (41.4 and 37.5 % in winter and spring, respectively); however, outdoors, higher DI was found in Cedros (64.1 %) than in Guadalupe (36.5 %) in winter, while in spring they presented similar DI. In both conditions (inside and outside the greenhouse), DI changed more from winter to spring in Guadalupe, which implies that this population has a higher seasonal or phenological sensitivity to low temperatures than Cedros.

Figure 1 Damage index (DI; mean ± standard error) caused by low temperatures (-12 °C, 4 h) on primary needles of Pinus radiata seedlings from Cedros (C) and Guadalupe (G) islands growing in greenhouse (a) and outdoors (b). Pairs of bars with different letters indicate statistically different DIs between populations (P = 0.05).
Larcher (2005) notes that differences in DI between ecotypes and between assessment periods are due to differences in physiological cold acclimatization capacity; that is, differences in carbohydrate metabolism, membrane characteristics and proteins associated with the freezing process (Pearce, 2001; Roden, Canny, Huang, & Ball, 2009). These include specific proteins that inhibit ice formation (anti-freeze proteins), reduce the rate of freezing, or protect the cellular protoplasm from the effects of temperature drop and dehydration, such as dehydrins (Ambroise et al., 2020; Larcher, 2005; Sharma & Deswal, 2014). Geographic origin has been shown to influence phenology, affecting the physiological condition of plants (Aldrete, Mexal, & Burr, 2008) and the synthesis of those molecules (Kreyling et al., 2012; Larcher, 2005). Several studies show that the two islands differ in ecological conditions, so they are considered two ecoregions (González-Abraham et al., 2010). In general, the environment on Guadalupe is more favorable in relative humidity because of the abundance of fog (Oberbauer, 2006; Perry, 2009), level of aridity and frequency of extreme temperatures (Hoeksema et al., 2012). These conditions, in addition to the geographical isolation, have differentiated the populations, to the extent that they are considered distinct varieties (Ledig et al., 1998; Rogers et al., 2006). In field trials, the population of Guadalupe shows greater growth and productivity than that of Cedros (Burdon, Bannister, & Low, 1992).
According to Table 2, subpopulations showed similar behavior within each population, with the exception of G2 outdoors, which had a lower DI than G1 in winter. In general, there is no evidence of adaptive differentiation between the subpopulations sampled on each island, despite differences in elevation, probably because there is still enough gene flow between them (Rogers, 2004). These results contrast with those described by Viveros-Viveros et al. (2009) in P. hartwegii Lindl. and Sáenz-Romero and Tapia-Olivares (2008) in P. devoniana Lindl. who found differences in DI associated with elevation of the site of origin.
Table 2 Damage index caused by low temperature (-12 °C, 4 h) in primary needles of seedlings from two subpopulations of Pinus radiata.
| Population | Subpopulation | Greenhouse | Outdoor | ||
|---|---|---|---|---|---|
| Winter | Spring | Winter | Spring | ||
| Cedros Island | C1 | 37.3 ± 8.5a | 30.6 ± 5.2 a | 64.8 ± 4.7a | 70.0 ± 9.7a |
| C2 | 45.6 ± 8.7a | 44.7 ± 4.2a | 63.5 ± 4.8a | 73.3 ± 8.8a | |
| Guadalupe Island | G1 | 57.5 ± 8.6a | 80.6 ± 6.8a | 49.8 ± 5.0a | 78.3 ± 9.6a |
| G2 | 62.3 ± 9.0a | 68.9 ± 7.6a | 24.3 ± 4.3b | 71.9 ± 10.3a | |
Mean ± standard error. Equal letters indicate that there are no significant differences between subpopulations (P = 0.05).
Effect of soil moisture
Figure 2 shows that soil moisture treatment affected populations differently in tolerance to low temperature damage. In the greenhouse, during the spring, the differences in damage between populations were more evident in drought than in favorable humidity conditions; moisture restriction increased slightly the DI in Guadalupe, but reduced it in Cedros. In winter, differences in the DI between populations were similar in the two moisture treatments. In seedlings exposed to weather during winter and spring, the differences in the DI between populations were not significant, regardless of the moisture treatment.
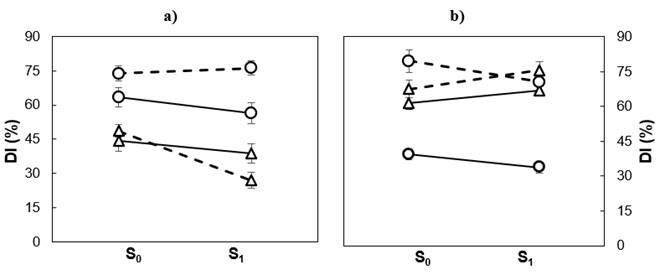
Figure 2 Soil moisture (S0 = irrigation; S1 = drought) and average damage index (DI) of Pinus radiata populations in winter (──) and spring (- - -) in seedlings from Cedros (() and Guadalupe (○) islands, growing in greenhouse (a) and outdoors (b).
Tolerance to frost and drought have much in common; in both, the basis of tolerance lies in the ability of the protoplasm to resist the dehydrating effect of direct or indirect water deprivation (Larcher, 2000). One of the metabolic processes involved includes the synthesis or accumulation of molecules involved in the osmotic adjustment, such as soluble sugars, proline and betaine (Ambroise et al., 2020; Arias, Bucci, Scholz, & Goldstein, 2015; Larcher, 2005). It is conceivable that such mechanisms are activated by exposure to severe short-term droughts; therefore, a pre-frost season drought could trigger low-temperature adaptation mechanisms with less damage (Kreyling et al., 2012; Walter, Jentsch, Beierkuhnlein, & Kreyling, 2013). In the greenhouse, this effect was recorded in the two populations during the winter and in Cedar population in spring; while outdoors it only occurred in Guadalupe, regardless of the period assessed. Studies in Quercus robur L. (Čehulić et al., 2019; Thomas & Ahlers, 1999) and in Q. petraea (Matt.) Liebl. (Thomas & Ahlers, 1999) point out that drought can increase frost susceptibility by modifying growth dynamics.
Absolute growth rate
For most measurement intervals, differences in AGR were significant (P ≤ 0.05) between treatments, between populations, and in the interaction of these factors under the two environmental conditions (Table 3). Differences between subpopulations were also found during some periods of growth in greenhouse, but not outdoors.
Table 3 Level of significance in each measurement interval (In) of the absolute growth rate (mm·d-1) in shoot length of two Pinus radiata populations. The seedlings were subjected to two moisture treatments in greenhouse and outdoors.
| Setting | Factor | GL | DF | I1 | I2 | I3 | I4 | I5 | I6 | I7 | I8 | I9 | I10 | I11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Greenhouse | T | 1 | 3 | * | ** | ns | ns | * | ** | ** | * | ns | * | * |
| P | 1 | 18 | ** | ** | ** | ** | ** | ** | * | * | ** | * | * | |
| TxP | 1 | 18 | * | * | * | ns | ** | * | * | * | ns | ** | * | |
| SP | 2 | 18 | ns | ns | ns | * | * | ns | * | * | ns | ns | * | |
| TxSP | 2 | 18 | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | |
| Outdoor | T | 1 | 3 | - | - | * | * | * | ** | ** | ** | ** | ** | * |
| P | 1 | 18 | - | - | ** | * | * | ** | ** | ** | * | * | * | |
| TxP | 1 | 18 | - | - | ns | ns | * | * | * | * | * | * | * | |
| SP | 2 | 18 | - | - | ns | ns | ns | * | ns | ns | ns | ns | ns | |
| TxSP | 2 | 18 | - | - | ns | ns | ns | ns | ns | ns | * | ns | ns |
T = moisture treatment, P = population, SP = subpopulation within population; GL = degrees of freedom of the numerator (factor); DF = degrees of freedom of the denominator (error); In = two-week interval from 26 October 2018 to 08 May 2019. *significant with P = 0.05 **significant with P = 0.01; ns = not significant.
The seedlings in S0 (irrigation) maintained a higher AGR than in S1 (drought) inside and outside the greenhouse, but the growth dynamics were different in both conditions, as shown in Figure 3. In the greenhouse there were several growth cycles; from day 82 after transplanting (DAT) the effect of moisture was noted with accelerated growth at S0, while at S1, growth was reactivated until after 112 DAT, then at 142 DAT it reached a AGR similar to that of S0 (2.5 mm·d-1) and, again, fell to values of 0.5 mm·d-1 at 164 DAT. Outdoors, plants in S1 maintained a slow growth phase (AGR < 1.0 mm·d-1) during the whole experiment, while in S0 they presented a low AGR only during the first part of winter, since at 89 DAT they reactivated growth until reaching a maximum AGR of 2.5 mm·d-1 at 133 DAT.

Figure 3 Absolute growth rate (AGR) of shoot length in greenhouse (a) and outdoors (b) by moisture treatment (S0 = irrigation; S1 = drought). DAT: Days after transplanting in greenhouse; (↓) dates when damage index was determined.
P. radiata is a polycyclic species that has evolved in a Mediterranean climate influenced by coastal humidity; in the juvenile stage, the species is adapted to grow all year round when the climate is suitable (Mead, 2013), but its growth dynamics is very sensitive to changes in soil moisture (Figure 3) and air temperature. In greenhouse, shoot growth never stopped and terminal bud formation was not observed, as it is in most temperate-cold pine species (Jansons, Matisons, Libiete-Zālīte, Baders, & Rieksts-Riekstiņš, 2013; Mead, 2013).
The different dynamics of shoot growth in seedlings and outside the greenhouse show sensitivity to changing environmental conditions. Outdoor seedlings were more exposed to low temperatures during winter (average minimum of 0.4 °C). This phenological variability in the shoot growth pattern, in response to changes in humidity and temperature, shows the complexity of cold tolerance in P. radiata, compared with other monocyclic growth pines such as P. pseudostrobus Lindl., P. montezumae Lamb. and P. hartwegii (Viveros-Viveros, Saenz-Romero, Lopez-Upton, & Vargas-Hernandez, 2007). Climent et al. (2009) and Bachofen et al. (2016) mention that it is more complex to interpret frost damage in pines of Mediterranean coastal climate, with multiple growth cycles and more sensitive to humidity and temperature fluctuations, such as P. radiata, than in species of continental alpine origin.
The Guadalupe population had higher AGR than Cedros in both environments, but the differences and the maximum peaks (2.6 mm·d-1 outdoors and 3.2 mm·d-1 in greenhouse) were greater in greenhouse (Figures 4a and 4b). Only Cedros subpopulations differed in AGR, especially in greenhouse (Figures 4c and 4d), since in Guadalupe subpopulations AGR was similar between them in both environments (Figures 4e and 4f). The differences in AGRs between populations can be attributed to genetic differences associated with the ecological conditions of the islands. Burdon et al. (1992) point out that, at the age of 12, the Cedros population had a lower rate of height growth than the Guadalupe population.
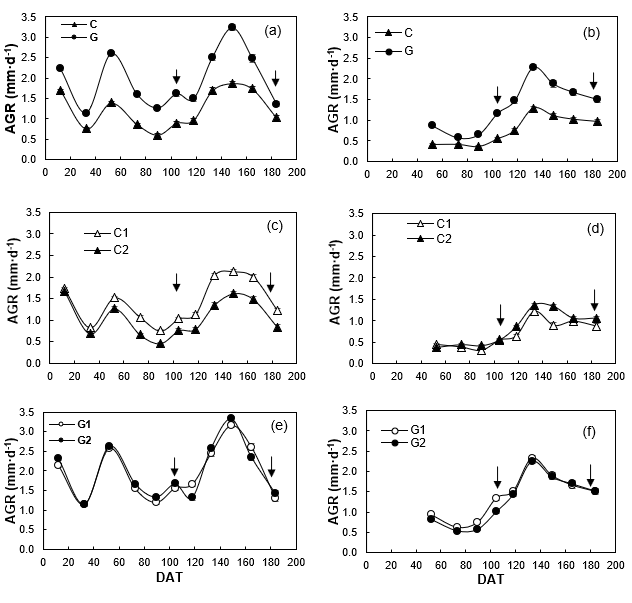
Figure 4 Absolute growth rate (AGR; mm·d-1) of shoot length of Pinus radiata of populations (a, b) and subpopulations (c, d, e, f) from Cedros (C) and Guadalupe (G) islands, under greenhouse (a, c, e) and outdoor (b, d, f) conditions. DAT: Days after greenhouse transplanting; (↓) dates when damage index was determined.
Relationship between DI and AGR
According to Figure 5, the correlation of DI with AGR was positive, but relatively low (r < 0.50). The highest correlations were obtained in winter (r ≥ 0.46), regardless of the growing environment; in contrast, in spring, the correlation was lower (0.25 ≤ r ≤ 0.28). Although the AGR was not clearly related to DI, the positive sign of the correlation indicates that seedlings with higher meristemic activity are more susceptible to low-temperature damage, as has been demonstrated in other conifers (Fløistad & Granhus, 2010; Sogaard, Granhus, & Johnsen, 2009). The positive relationship between DI and AGR was more evident in favorable wet conditions than in dry conditions.

Figure 5 Correlation between the damage index (DI) in primary needles and the absolute growth rate (AGR) in winter (a and b) and spring (c and d) in Pinus radiata seedlings from the Cedros (triangles) and Guadalupe (circles) islands with favorable soil moisture (black) and drought (white), under greenhouse (a and c) and (b and d) outdoor conditions.
When the correlation analysis was done for each moisture treatment (n = 16), the correlation was higher at S0 than at S1 (Figure 6). The correlation increases (r = 0.77) if only data from Guadalupe subpopulations are considered (n = 8) or if the data from the two Cedros subpopulations in winter (n = 14) are excluded, because their seedlings practically did not grow in this period. Drought drastically reduced the AGR and altered the relationship between DI and AGR. This explains, in part, the differences observed in DI between populations regarding the rate and seasonal dynamics of shoot growth inside and outside the greenhouse. The Cedros stock has lower intrinsic AGR than Guadalupe and also shows higher tolerance to frost damage, results that are consistent with previous information (Mead, 2013). The greater sensitivity of Guadalupe population to the effect of soil moisture in DI (Figure 2) and in the shoot growth dynamics (Figure 5) implies that there are important differences between populations in the response and adaptation mechanisms to water stress, as a result of local adaptation processes on the islands where they have evolved.
Conclusions
The populations of Pinus radiata on Cedros and Guadalupe Islands differ markedly in their tolerance to low temperatures and in the rate and dynamics of shoot growth inside and outside the greenhouse. The population of Guadalupe island is more sensitive in the damage index (DI) as a function of the environment, due to the changes in its growth dynamics when conditions change. The population of Cedros island had a lower growth rate and lower DI, due to its adaptation to the more adverse environment in which it has evolved. There is a positive relationship between DI and absolute growth rate, especially in favorable soil moisture conditions. The differences in productivity and tolerance to low temperatures of these populations are very useful in the domestication of P. radiata in the current context of climate change, so the efforts of in situ conservation of these genetic resources become more relevant.











 texto en
texto en 



