Introduction
The term mycorrhiza refers to the symbiotic association between plant roots and the fungal mycelium of the soil (Honrubia, 2009). It is estimated that 90 % of plants form mycorrhizal symbiosis; the development of this type of symbiosis began approximately 400 million years ago (Sosa, Sanchez, Morales, & Cruz, 2006). Mycorrhizae promote the solubility and mobility of nutrients from the soil to the plant, conferring greater resistance to water stress and damage by disease (Carrasco-Hernández et al., 2010; Pera & Parladé, 2005). Likewise, mycorrhizae improve soil structure and interaction with beneficial microorganisms, thus contributing to plant development and growth (Gómez-Romero, Villegas, Sáenz-Romero, & Lindig-Cisneros, 2013; Osorio, 2012).
Among the types of mycorrhizae are endomycorrhizae and ectomycorrhizae; the latter are characterized by the formation of external mycelium and a Hartig network between the root cells without penetrating the cell wall. Ectomycorrhiza is established in more than 5 000 species of fungi, mostly in the class Basidiomycetes, and in about 3 000 species of angiosperms and gymnosperms. Ectomycorrhizae are associated with forest species and are relevant in the production of plants of the genus Pinus in nurseries. This genus is a forced symbiont, so the absence of mycorrhizae affects plant survival and development (García, Sarmiento, Sierra, & Mejía, 2012). Hence the importance of considering the presence of ectomycorrhizae as a parameter that evaluates the quality of the forest plant produced in the nursery (Martínez, Sarmiento, Sigala, Rosales, & Montoya, 2016; Secretaría de Economía, 2016).
In the nursery stage, the production of forest seedlings in containers requires inputs and processes to favor the quality of the plant material. Some of these are the growth medium, the type of container, the incorporation of fertilizers, inoculation with mycorrhizal fungi, irrigation, and control of environmental factors (Baltasar, Barroetaveña, & Rajchenberg, 2007; Castro-Garibay, Aldrete, López-Upton, & Ordáz-Chaparro, 2018). Nutrients are added either by incorporating controlled-release fertilizers into the substrate and nutrient solutions into the irrigation water, according to the needs of the plant during the production process, or by using both options (Dumroese, Landis, & Wilkinson, 2012). Respect to ectomycorrhizal fungi, the simplest and most common way to inoculate spores into plants is through water during irrigation (Baltasar et al., 2007).
Within the forest plant production cycle in nursery, it is more common for applied commercial inoculants to contain a mixture of spores of several native or exotic fungal species, such as Pisolithus tinctorius (Pers.) Coker et Couch and Scleroderma citrinum Pers. (García et al., 2012). In this regard, Prieto, García, Mejía, Huchín, and Aguilar (2009) found that out of 10 forest nurseries in the state of Durango only one considered mycorrhization as a cultural practice, using commercial inoculants with the spores of the exotic fungi mentioned above.
Three trends have been reported regarding mycorrhization and fertilization. The first refers to an adverse relationship, where the higher the fertilization, the lower the degree of mycorrhization (Baltasar et al., 2007; Salgado, Rajchenberg, & Barroetaveña, 2009). The second reports that mycorrhization is not affected by fertilization (Khasa et al., 2001) and the last one sustains a differentiated response that depends on the species of the ectomycorrhizal fungus; that is, one species may be more susceptible to fertilization than another (Brundett, Bougher, Dell, Grove, & Malajczuk, 1996; Trappe, 1977).
In Mexico, Escobar-Alonso and Rodríguez (2019), in their review of the state of the art on plant quality in Pinus, indicate the scarcity of studies related to mycorrhizal species, levels of mycorrhization and their effect on plant quality, and therefore recommend the increase of this type of trials. In this sense, the aim of the present work was to evaluate the effect of two commercial mycorrhizal inoculants and a controlled-release fertilizer on the development of Pinus engelmannii Carr. in nursery. One commercial inoculant contained a mixture of spores of fungi native to Mexico (Amanita rubescens Pers., Amanita sp., Lactarius indigo [Schwein] Fr., Ramaria sp. and Boletus sp.), while the other included a mixture of spores of exotic fungi (P. tinctorius and S. citrinum). It is assumed that at least one combination of inoculum type and fertilization dose favors the plant quality of P. engelmannii to a greater extent.
Materials and methods
Location of the study area
The experiment was conducted at the Praxedis Guerrero forest nursery of the Secretaría de Recursos Naturales y Medio Ambiente of the State of Durango, Mexico. The nursery is located at kilometer 12.5 of the Durango-El Mezquital highway, southeast of the city of Durango, Dgo. at the coordinates 23° 56' 58.3" LN and 104° 34' 07.4" LO at 1 890 m.
Production conditions
According to its development phase, the plant grew in different areas of the nursery. In the establishment stage, from October 11th to November 21st, 2018, the plant was in a greenhouse with a metallic structure protected with a 720 µm white polyethylene film, covered by a 50 % shadow mesh and with side curtains. From 22 November 2018 to 31 January 2019, the plant continued to grow in a tunnel greenhouse with green polyethylene, 720 µm caliber and 50 % shadow mesh. The average temperature and relative humidity were 20 °C and 66 %, respectively.
Three and a half months after sowing (from February 1st to May 11th 2019), corresponding to the fast growing stage, the vegetative material was moved to another production area with 50 % shadow mesh, where the average temperature was 18.5 °C and relative humidity 48 %. Finally, in the pre-conditioning stage, seven months after sowing (from May 12th to June 12th 2019), the plant was exposed to outdoor conditions with an average temperature of 23 °C and relative humidity of 42 %.
Plant production
Before sowing, as a pre-germination treatment, the seed was soaked in water at room temperature for 24 h and at the end fungicide was applied. The sowing was carried out on October 11th, 2018. A mixture of peat moss (57 %), vermiculite (23 %) and agrolite (20 %) was used as substrate. The total porosity of the substrate was 68.1 % and the aeration porosity was 31.5 % with a water retention capacity of 36.6 %, normal values according to the NMX-AA-170-SCFI-2016 standard (Secretaría de Economía, 2016). Black containers (165 mL volume) made of rigid polyethylene with internal root guides were used. During the preparation of the substrate, the nutrition of the plant was done by adding granulated controlled-release fertilizer (from eight to nine months) with a composition of 11-28-11 of N-P-K, respectively.
Prior to inoculation, spore concentrations in each inoculum were checked with the Neubauer chamber or hematocytometer to determine the doses used in each proposed treatment. Inoculants with native and exotic fungal spores had a concentration of 1.05 x 107 spores-mL-1 and 6.5 x 106 spores-mL-1, respectively. Inoculation was carried out four months after sowing when the seedlings had secondary roots (Figure 1a). The procedure consisted in injecting the spore solution directly into the growing substrate, at an angle of approximately 45° to the main root of the plant at a depth of 5 cm, ensuring that the solution had the greatest possible contact with the root system (Figure 1b).
Evaluated treatments and experimental design
The treatments were distributed in a randomized full-block experimental design. Nine treatments were evaluated considering controlled inoculation derived from two commercial products plus a non-inoculation condition, combined with two doses of controlled-release fertilizer, as well as a control without fertilizer (Table 1). A commercial inoculum was used which consisted of a mixture of spores from fungi native to Mexico: A. rubescens, Amanita sp., L. indigo, Ramaria sp. and Boletus sp.; the other commercial inoculum included a mixture of spores from exotic fungi: P. tinctorius and S. citrinum. Each treatment consisted of four replicates with 49 plants each.
Table 1 Treatments applied with commercial mycorrhizal inoculants and controlled-release fertilizer in the development of Pinus engelmannii in nursery.
| Treatments | Description | |
|---|---|---|
| 1 | (Control) | No controlled-release fertilizer and no inoculant |
| 2 | (FB) | Low fertilization (3 g·L-1) |
| 3 | (FA) | High fertilization (6 g·L-1) |
| 4 | (ICHN) | Commercial inoculant with native fungal spores (1 mL·plant-1 [1.05 x 107 spores·mL-1]) |
| 5 | (ICHE) | Commercial exotic fungal spore inoculant (1.5 mL·plant-1 [6.5 x 106 spores·mL-1]) |
| 6 | (ICHN+FB) | Commercial inoculant with native fungal spores plus low fertilization |
| 7 | (ICHN+FA) | Commercial inoculant with native fungal spores plus high fertilization |
| 8 | (ICHE+FB) | Commercial inoculant with exotic fungal spores plus low fertilization |
| 9 | (ICHE+FA) | Commercial inoculant with exotic fungal spores plus high fertilization |
Variables evaluated
At eight months after sowing and four months after inoculation, the survival rate of the lot under study was determined, and from a total of 10 seedlings per experimental unit, extracted at random: stem height, root collar diameter, wet and dry biomass of the aerial part, and root were evaluated. To estimate the dry biomass, the plants were dehydrated at 65 °C in a forced ventilation oven for 72 h. Three plant quality indices were also determined which, through an evaluation of morphological variables, allow the prediction of the adaptation opportunity of a plant when it is moved to the field (Prieto et al., 2009). These indices were: ratio of dry biomass of the aerial part/dry biomass of the root part, lignification index (IL) and Dickson quality index (ICD) (Dickson, Leaf, & Hosner, 1960). The last two were obtained using the following expressions:
IL =
The percentage of mycorrhizal colonization (PCM) was evaluated in the root system of three seedlings taken randomly from each experimental unit. The substrate adhered to the root system of the plants was removed with running water; subsequently, 100 cm of secondary roots per plant were randomly selected and preserved in a solution of formaldehyde, alcohol (96°), glacial acetic acid and distilled water in a ratio of 10:50:5:35. The number of mycorrhized and non-mycorrhized apexes was determined with a stereo microscope (Leica® EZ4 HD, Switzerland). The PCM was calculated with the equation used by García (2018):
The percentage values of mycorrhizal colonization and survival were transformed with the arcsine and square root function. The data were subjected to an analysis of variance; in cases where significant differences existed, a Tukey mean separation test was performed, using the SAS® statistical program version 9.2 (Statistical Analysis System, 2009).
Results and discussion
Morphological variables
The average value of the root collar diameter showed statistical differences (P ≤ 0.05) between the treatments. The difference between the extreme values (2.60 to 5.11 mm) was of 100 %; according to Figure 2, the commercial exotic fungal spore inoculum (1.5 mL·plant-1) plus low fertilization (3 g·plant-1) (treatment 8 = ICHE + FB) generated the highest values. NMX-AA-170-SCFI-2016 stipulates that the P. engelmannii plant requires a minimum diameter of 5 mm to be considered as a quality plant that can be planted in the field (Secretaría de Economía, 2016).

Figure 2 Diameters of Pinus engelmannii obtained in treatments with commercial mycorrhizal inoculants and controlled-release fertilizers in nursery. FB = low fertilization (3 g·L-1), FA = high fertilization (6 g·L-1), ICHN = commercial inoculant with native fungal spores (1 mL·plant-1 [1.05 x 107 spores·mL-1]), ICHE = commercial inoculant with exotic fungal spores (1.5 mL·plant-1 [6.5 x 106 spores·mL-1]).
Although treatments 2 (FB), 3 (FA) and 9 (ICHE + FA) were statistically equal (P ≤ 0.05) to treatment 6 (ICHN + FB), only the latter managed to comply with the minimum diameter established by NMX-AA-170-SCFI-2016; while the plants in the control treatment and the unfertilized treatments, regardless of the type of inoculum, had the lowest response (Figure 2).
Figure 3 shows the effect of treatments on the dry biomass of plants. The treatments with the highest total dry biomass were 6 (ICHN + FB) and 8 (ICHE + FB) with significant differences (P ≤ 0.05) with respect to the rest of the treatments. The dry biomass of the aerial part varied from 0.36 to 1.67 g, with treatment 8 (ICHE + FB) once again standing out. Fertilizer treatments and those that combined inoculum and fertilizer produced more aerial biomass than the solely inoculated and control treatments. Regarding the dry biomass of the root part, the range was from 0.44 to 1.12 g, where the treatment of the commercial inoculant with native fungal spores (1 mL·plant-1) plus low fertilization (3 g·plant-1) (treatment 6 = ICHN + FB) stood out; the lowest values occurred in the unfertilized treatments.
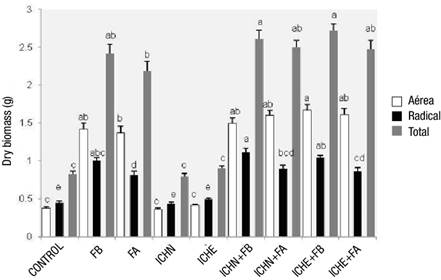
Figure 3 Dry biomass of Pinus engelmannii in the nursery, under treatments with commercial mycorrhizal inoculants and controlled-release fertilizers. FB = low fertilization (3 g·L-1), FA = high fertilization (6 g·L-1), ICHN = commercial inoculant with native fungal spores (1 mL·plant-1 [1.05 x 107 spores·mL-1]), ICHE = commercial inoculant with exotic fungal spores (1.5 mL·plant-1 [6.5 x 106 spores·mL-1]).
Rentería-Chávez, Pérez-Moreno, Cetina-Alcalá, Ferrera-Cerrato, and Xoconostle-Cázares (2017) determined that Pinus greggii Engelm. plants inoculated with edible ectomycorrhizal fungi developed higher total biomass than those without inoculation. However, in the present study, the treatments with the lowest results were not the non-inoculated ones, but those without fertilizer. This is consistent with the findings of Vázquez-Cisneros et al. (2018), who found that the addition of slow-release fertilizer positively influenced the growth of species in the genus Pinus compared to the unfertilized plant.
Plant quality indices
The mean values of the ratio of dry biomass of the aerial part to dry biomass of the root part varied from 0.86 to 1.94. Treatments 3 (FA), 7 (ICHN + FA), 8 (ICHE + FB), and 9 (ICHE + FA) were in the range of 1.5 to 2.5, which represents an appropriate balance between transpiration and water uptake areas (Sáenz, Muñoz, Pérez, Rueda, & Hernández, 2014). Treatments 1 (Control), 4 (ICHN) and 5 (ICHE), characterized by not having fertilizer, obtained lower values (Table 2), which means that the root biomass is higher than the aerial one; therefore, there is disproportionality in the plant (Rodríguez, 2008).
Table 2 Mean values of plant quality indices of Pinus engelmannii at eight months after sowing, under mycorrhizal inoculation and controlled-release fertilization treatments.
| Treatments | Relation dry biomass air/radical | Index of lignification (%) | Dickson’s quality index | |
|---|---|---|---|---|
| 1 | Control | 0.86 ± 0.04 | 25.76 ± 1.04 bc | 0.36 ± 0.02 d |
| 2 | FB | 1.41 ± 0.07 | 25.11 ± 0.49 bc | 0.85 ± 0.04 abc |
| 3 | FA | 1.76 ± 0.09 | 22.84 ± 0.60 c | 0.70 ± 0.05 c |
| 4 | ICHN | 0.87 ± 0.04 | 27.85 ± 0.81 ab | 0.30 ± 0.02 d |
| 5 | ICHE | 0.89 ± 0.06 | 30.56 ± 0.87 a | 0.36 ± 0.02 d |
| 6 | ICHN+FB | 1.38 ± 0.06 | 24.89 ± 0.41 bc | 0.98 ± 0.05 a |
| 7 | ICHN+FA | 1.89 ± 0.09 | 23.91 ± 0.68 c | 0.76 ± 0.04 bc |
| 8 | ICHE+FB | 1.63 ± 0.07 | 25.10 ± 0.47 bc | 0.90 ± 0.04 ab |
| 9 | ICHE+FA | 1.94 ± 0.10 | 22.99 ± 0.54 c | 0.76 ± 0.04 bc |
Means with different letters in the same column indicate significant difference between treatments according to Tukey (P ≤ 0.05). ± = Standard error of the media. FB = low fertilization (3 g·L-1), FA = high fertilization (6 g·L-1), ICHN = commercial inoculant with native fungal spores (1 mL·plant-1 [1.05 x 107 spores·mL-1]), ICHE = commercial inoculant with exotic fungal spores (1.5 mL·plant-1 [6.5 x 106 spores·mL-1]).
Prieto et al. (2009) refer that the lignification index expresses the level of pre-conditioning of the plants; when they are of quality, they present values of 25 to 30 %. This is related to the weathering phase where individuals suffer stress due to the drastic change in light conditions, from shadow mesh to outdoor and from more to less availability of humidity. In the present study, the treatments that lacked fertilizer reached optimal values (P ≤ 0.05).
The Dickson’s Quality Index is an integrated measure of morphological traits, where high values express better plant quality (García, 2018; Prieto et al., 2009). This index has been used to select better proportioned plants, as well as to predict their response once planted in the field (Sáenz et al., 2014). The results varied from 0.30 to 0.98, positioning the commercial inoculant with native fungal spores treatment (1 mL·plant-1) plus low fertilization (3 g·plant-1) (treatment 6 = ICHN + FB) as the best, with significant differences (P ≤ 0.05) with respect to the rest of the treatments. This is in agreement with what Martínez et al. (2016) report, who obtained an index of 1.08 as an effect of the inoculation with Russula delica Fr. in P. engelmannii, maintaining a fertilization regime according to the stages of development of the plant. According to this parameter, in the present study, the plants in the treatments without fertilization were those of lower quality (Table 2).
Survival
According to Figure 4, survival varied from 80 to 96 %, with no significant differences (P ≤ 0.05) among treatments. The literature reports negative effects on plants when using high doses of fertilization (Marschner, 2012), favoring plant susceptibility to pests and diseases that lead to death. This may explain the mortality caused by a more severe damping off outbreak in treatments 3 (FA), 7 (ICHN + FA) and 9 (ICHE + FA), characterized by high fertilization doses. This disease occurs in forest nurseries and is caused by a complex of fungi of the genera Phytopthora, Pythium, Fusarium and Rhizoctonia; its main characteristic is the necrosis of the conduction tissue at the neck of the seedling root that causes its death (Cibrián, Alvarado, & García, 2007).

Figure 4 Survival of Pinus engelmannii plants in treatments with commercial mycorrhizal inoculants and controlled-release fertilizer, after eight months from sowing. FB = low fertilization (3 g·L-1), FA = high fertilization (6 g·L-1), ICHN = commercial inoculant with native fungal spores (1 mL·plant-1 [1.05 x 107 spores·mL-1]), ICHE = commercial inoculant with exotic fungal spores (1.5 mL·plant-1 [6.5 x 106 spores·mL-1]).
Percentage of mycorrhizal colonization
Figure 5 shows that the PCM varied from 15 to 71 % and showed a reverse trend to the fertilization dose used. Treatments 3 (FA), 7(ICHN + FA) and 9 (ICHE + FA), characterized by having doses of 6 g·L-1 of controlled-release fertilizer, had low mycorrhization regardless of the type of inoculant, which is consistent with what is reported by Baltasar (2007) and Salgado et al. (2009). The treatments with higher PCM were 1 (Control), 4 (ICHN), 5 (ICHE) and 6 (ICHN + FB), highlighting treatment 4, although without statistical differences between them, indicating that the symbiosis was established with more abundance in a nutrient limited environment, functioning as an auxiliary nutrition mechanism. This is consistent with the observations of Bücking, Liepold, and Ambilwade (2012), who recognize the existence of two pathways for nutrient absorption in plants; the direct one involving the epidermis and root hairs, and the second through the ectomycorrhizae. In the latter, the external mycelium favors a greater exploration in the soil. Regarding the treatments without inoculation, due to the test being conducted under normal production conditions in nursery, the plants developed undesirable ectomycorrhizal colonization.
Although there was a negative trend with respect to the percentage of mycorrhization and the fertilization used, the results showed that treatment 6 (ICHN + FB) had a balance between the two variables; this had a favorable impact on the quality of the plant produced in that treatment, confirming the hypothesis. In general, the fungus-root association had a differentiated response depending on the amount of fertilizer used, which coincides with that proposed by Trappe (1977). In this respect, Brundett et al. (1996) carried out a study on fertilization and mycorrhizal fungi associated with Eucalyptus diversicolor F. Muell., and found that the increase in the dose of mineral fertilization had an effect on the decrease in mycorrhizal colonization; however, this effect was differential, finding that the genus Scleroderma is less susceptible to fertilization than P. tinctorius.

Figure 5 Mycorrhizal colonization (PCM) in Pinus engelmannii plants under mycorrhizal treatments and controlled-release fertilizers, after eight months from sowing. FB = low fertilization (3 g·L-1), FA = high fertilization (6 g·L-1), ICHN = commercial inoculant with native fungal spores (1 mL·plant-1 [1.05 x 107 spores·mL-1]), ICHE = commercial inoculant with exotic fungal spores (1.5 mL·plant-1 [6.5 x 106 spores·mL-1]).
Characterization of ectomycorrhizae
In the present study three morphotypes of ectomycorrhizae were found: one from the commercial inoculant with native fungal spores and two from the commercial inoculant with exotic fungal spores. From the first inoculant containing spores of A. rubescens, Amanita sp., L. indigo, Ramaria sp. and Boletus sp., only one morphotype was identified that was related to the genus Amanita spp. and presented dichotomous branching without rhizomorphs, with straight unbranched ends of cylindrical non-inflammatory shape and slightly sharp end, ochre, yellowish ochre or white with presence of mantle (Figure 6a) (Determination of ectomycorrhizae [DEEMY], 2019). The remaining species contained in the inoculant (L. indigo, Ramaria sp. and Boletus sp.) did not form ectomycorrhizae. In this regard, Ishida, Nara, Tanaka, Kinoshita, and Hogetsu (2008) and Nara (2009), mentioned that some species of ectomycorrhizal fungi are pioneers, so their spores respond quickly to germination in the presence of roots of host plants, in contrast, other species (which include species of the genus Lactarius) require additional stimuli obtained as the habitat develops, so the spore inoculation may not be successful.
From the inoculant with exotic fungal spores, two morphotypes were identified; the first one related to S. citrinum, which in structures with initial development was characterized by ectomycorrhizae with stipe and dichotomous branching, white color and without presence of rhizomorphs. The mycorrhizal systems in mature stages presented mainly stipe and coralloid branching of yellowish-white color, with straight unbranched ends and presence of rhizomorphs (Figure 6b). The second morphotype had stipe and coralloid structures with brown dichotomous ramifications, straight unbranched apexes and rhizomorphs of restricted connection to the lateral part of the apexes. This description corresponds to the one made by Valdés, Ambriz, Camacho, and Fierros (2010), who tested the same product used in the present work and which also contained spores of P. tinctorius, only in roots of Pinus devoniana Lindl. (Figure 6c).
In treatments without inoculum (Control, FB and FA) mycorrhizal colonization was observed in initial stages, a condition that did not allow the identification of species. In general, mycorrhizal systems in the treatments with only inoculum (ICHN and ICHE), as well as those with inoculum and low fertilization (ICHN + FB and ICHE + FB) showed ectomycorrhizae in mature stages; in contrast, treatments with inoculum and high fertilization (ICHN + FA and ICHE + FA) showed mycorrhizal structures in initial stages of development (Figures 6d and 6e). This could indicate that the level of mycorrhization, up to the time of evaluation, was affected as a function of fertilization dose, delaying the development of ectomycorrhizae, as observed by Marx (1980).

Figure 6 Ectomycorrhizal morphotypes in Pinus engelmannii: (a) Amanita spp. related morphotype; (b) Scleroderma citrinum related morphotype; (c) Pisolithus tinctorius related morphotype described by Valdés et al. (2010); (d) initial stage ectomycorrhiza; (e) mature stage Scleroderma citrinum related ectomycorrhiza.
Conclusions
The combination of commercial inoculant with native fungal spores (1 mL·plant1) and 3 g·L-1 fertilization led to a better quality of the plant compared to commercial inoculant with exotic fungal spores (1.5 mL·plant1), demonstrating that both components favor the quality of Pinus engelmannii in nursery. The incorporation of the high dose of controlled-release fertilizer led to a negative trend in the percentage of mycorrhization. The diversity of ectomycorrhizal fungal spores contained in commercial inoculants does not result in a diversity of mycorrhizal formation, as it happens with the commercial inoculant of native fungi. The results represent a contribution to the technical knowledge for the production of quality plant of P. engelmannii in nursery, which affects the survival of the species in the field.











 texto en
texto en 



