Introduction
A substrate called "mezcla base” (base mixture) composed of peat moss, vermiculite and perlite at a 3:1:1 ratio has been used in Mexican forest nurseries. This mixture is widely used as a substrate with physical and chemical characteristics suitable for plant production; however, the main components are imported at high costs, which directly impacts the cost of production. Currently there are alternative substrates prepared from materials such as pine bark and sawdust, coconut fiber or rice husk that can replace the base mixture for plant production and reduce its unit cost in forest nurseries (Aguilera-Rodríguez, Aldrete, Martínez-Trinidad, & Ordaz-Chaparro, 2016).
Plant quality is determined by genetic, morphological, physiological and health characteristics; in the latter, phytopathogenic agents can have an economic impact (Villar, 2003). Fusarium circinatum Nirenberg & O´Donnell (teleomorph = Gibberella circinata) occurs in pine plantations and is the causal agent of pitch canker. In nurseries, this fungus causes damping-off, root rot before and after emergence, wilting and rotting of the neck and stem in pine seedlings (García-Díaz et al., 2017; Herron et al., 2015). The control of this pathogen is complicated and costly since chemical treatments are mainly used that, besides endangering the natural balance and human health (Cubillos, Páez, & Mejía, 2011; Solano-Bonilla, & Brenes-Chacón, 2012), cause phytotoxicity and resistance, resulting in an increase in dose and number of applications (Reglinski & Dick, 2005; Romero, Crosara, & Baraibar, 2008).
An alternative for controlling F. circinatum is the use of biological control agents such as the fungus Trichoderma (Okorski, Oszako, Nowakowska, & Pszczólkowska, 2014). The species most used in biocontrol are T. virens Mater., T. viride Pers. and T. harzianum Rifai, the latter being the antagonist fungus with the greatest commercial use and the most researched for its application in biological control (Benítez, Rincón, Limón, & Codón, 2004; Harman, Björkman, Ondik, & Shoresh, 2008). In New Zealand, Cummings et al. (2016) conducted a study at nursery and plantation level in which they analyzed trays, substrates and soils; the researchers isolated and identified, by morphology and phylogenetic analysis, 16 native species of Trichoderma with the possibility of control for phytopathogens.
The mechanisms of action of Trichoderma on phytopathogenic fungi are mainly competition for nutrients and the production of antibiotics, such as trichodermin, suzukacillin, alamethycin, dermadin, trichothecenes and trichorzianin, whose function is to inhibit spore germination (Infante, Martínez, González, & Reyes, 2009). The antagonist increases its rate of development by covering the surface and preventing the establishment of the pathogen by inducing resistance in plants (Desender, Andrivon, & Val, 2007). In the interaction with the roots, Trichoderma spp. confers benefits such as the promotion of growth and the increase of systemic resistance, having effects on the development and productivity of the plant (Benítez, Rincón, Limón, & Codón, 2004; Dumroese, Kim, & James, 2012).
In Mexico, the application of T. harzianum as a Fusarium antagonist is common in forest nurseries; however, little is known about the effect and efficacy of the antagonist when incorporated into different substrates. Therefore, the objective of this study was to evaluate the pathogenicity and incidence of F. circinatum in P. greggii seedlings planted in three substrates and the use of T. harzianum as a biocontrol mechanism against the pathogen.
Materials and methods
Substrates and seed
The experiment was carried out in the greenhouses (19˚ 29´ 34” N and 98˚ 53´ 38” W, elevation of 2 240 m) of the Division of Forest Sciences (DiCiFo) of the Universidad Autónoma Chapingo in Texcoco, Mexico.
The substrates evaluated were as follows: S1 = peat moss (PROMIX®), agrolite and vermiculite; S2 = pine sawdust, pine bark and peat moss; and S3 = pine bark, pine sawdust and peat moss, all at a 60:20:20 ratio (Hernández-Zarate, Aldrete, Ordaz-Chaparro, López-Upton, & López-López, 2014). The sawdust was obtained from a local sawmill with fresh material (no more than 15 sawing days) of Pinus patula Schltdl. & Cham. Composted Pinus douglasiana Martínez bark was obtained from the MASVI company in the southern region of Jalisco. Osmocote Plus® 15-9-12 controlled-release fertilizer was added to the mixtures of each substrate in doses of 7 g·L-1, with a release time of eight to nine months.
Deepot trays with 42 cavities and 170 mL cells were used. The P. greggii seed was obtained from a germplasm bank located in the community of Pueblo Nuevo, municipality of Chignahuapan, Puebla (19° 52´ 60" N and 98° 06´ 36" W and elevation of 2 450 m). After filling the cells with each type of substrate, sowing was done directly by placing two seeds per cavity. A month later, thinning was done to leave only one seedling per cavity and, at the end of May, when the plants were 45 days old (May 25, 2015), the inoculation with F. circinatum and the first application of T. harzianum to the substrate were carried out. Irrigation was applied daily to the surface during the germination and emergence of the plants, then every other day. The experiment lasted 13 weeks (June to September).
Pinus greggii plants inoculated with Fusarium circinatum
The P. greggii plants were inoculated with the SF5 strain isolated from the Military Forest Nursery of Atlangatepec, Tlaxcala, and obtained from the DiCiFo Forest Parasitology laboratory strain repository. The strain is identified, morphologically and with three molecular markers, as F. circinatum; its accession numbers deposited in the GenBank are ITS (KX276596), TEF (KZ337005) and IGS (KX306890). For the inoculation of F. circinatum (Fc), pure strains were taken with eight days of growth in PDA culture medium with development of mycelium and sporulation of the fungus. The phytopathogen was applied by injection of 20 mL of a suspension of 7.9 x 104 spores·mL-1 (García-Díaz et al., 2017) to the substrate (S) of each cell. The treatments inoculated with F. circinatum were T2 (S1+Fc), T6 (S2+Fc) and T10 (S3+Fc) (Table 1).
Table 1 Treatments used in the production of Pinus greggii in individual 170 mL cells.
| Treatment number | Treatments |
|---|---|
| T1 | S1 + Fusarium circinatum (Fc) + Trichoderma harzianum (Th) |
| T2 | S1 + Fc |
| T3 | S1 + Th |
| T4 | S1-C (uninoculated) |
| T5 | S2 + Fc + Th |
| T6 | S2 + Fc |
| T7 | S2 + Th |
| T8 | S2-C |
| T9 | S3 + Fc + Th |
| T10 | S3 + Fc |
| T11 | S3 + Th |
| T12 | S3-C |
S1: peat moss, perlite and vermiculite; S2: pine sawdust, pine bark and peat moss; S3: pine bark, pine sawdust and peat moss. The ratio of materials in the three mixtures was 60:20:20.
Pinus greggii plants inoculated with Trichoderma harzianum
The PHC® T. harzianum (Th) commercial strain T-22 KRL-AG2 was used in doses of 3.4 g·L-1 of water, with a dilution of 107 spores·g-1 of dry weight. Inoculation was performed by applying 20 mL per cell, by injection to the substrate, in three applications. The first application of Th was made on May 25 at the time of setting up the experiment. The other two applications were made at monthly intervals (June 25 and July 25, 2015). The treatments inoculated with T. harzianum were T3 (S1+Th), T7 (S2+Th) and T11 (S3+Th) (Table 1).
Pinus greggii plants inoculated with Fusarium circinatum and Trichoderma harzianum
In the first application, a mixture of 10 mL of F. circinatum and 10 mL of T. harzianum (Fc+Th) was used with the same concentrations and doses used in the individual treatments. Subsequently, two other applications were made (one each month) with only T. harzianum. The mixture was injected into the corresponding cells of each substrate. The treatments inoculated with the mixture were T1 (S1+Fc+Th), T5 (S2+Fc+Th) and T9 (S3+Fc+Th) (Table 1). In each substrate, treatment without application of fungi was used as a control: T4 (S1-C), T8 (S2-C) and T12 (S3-C) (Table 1).
Variables evaluated
Pathogenicity and incidence of Fusarium circinatum
The pathogenicity of the F. circinatum strain was determined by its ability to cause disease, by weekly recording of typical damping-off symptoms and root rot. At the end of the experiment, some root portions of the diseased seedlings were sown in PDA culture medium with streptomycin sulphate (0.05 mg·L-1), for the re-isolation of the pathogen and corroboration of Koch's postulates. The incidence of F. circinatum was evaluated weekly and the accumulated percentage of diseased plants was obtained. Incidence and pathogenicity were evaluated for 13 weeks from mid-June to late September 2015.
The effect of the antagonist fungus on incidence and pathogenicity was evaluated with the mixture of F. circinatum plus T. harzianum (Fc+Th) in the three substrates.
Morphological indices
Morphological indices were evaluated in treatments inoculated with T. harzianum (T3: S1+Th, T7: S2+Th and T11: S3+Th) and uninoculated (T4: S1-C, T8: S2-C and T12: S3-C). The evaluation was carried out at the end of October 2015, six months after sowing. The study variables were: stem diameter (D), measured in the section where it differs from the main root; height of the aerial part (H), obtained from the point where the diameter of the stem was measured up to the apex of the terminal bud; root dry weight (RDW) and dry weight of the aerial part (ADW), determined with an analytical balance (OHAUS, model Galaxy 200), after dehydration of the plants in a drying oven (FELISA, FE-143) at 70 °C for 72 h. The ratio comparing the aerial dry weight to the root dry weight (ADW/RDW) was also evaluated, along with the slenderness index (SI), obtained by dividing the plant height (cm) by the diameter value (mm), and the Dickson quality index (DQI), obtained with the equation TDW/(H/D) + (ADW/RDW) where TDW is the total dry weight of the plant (Dickson, Leaf, & Hosner, 1960).
Experimental design and statistical analysis
The experimental design was completely random, which was represented by the following model:
Yij = µ + Ti + eij
where,
Yij |
variable response in the j-th repetition of the i-th treatment |
µ |
total effect of the mean |
Ti |
effect attributed to the i-th treatment |
Eij |
random component error |
Three substrates (S1, S2 and S3) were used with and without F. circinatum (Fc), and with and without T. harzianum (Th), for a total of 12 treatments with four replications (Table 1). Each replication was a 42-cavity deepot tray; the experimental unit consisted of the 20 central plants (80 P. gregii seedlings per treatment, giving a total of 960 seedlings evaluated). Morphological standards were measured in plants with and without Th in the three substrates; the experimental unit was made up of 15 plants randomly selected from the central part of each deepot tray (60 P. gregii seedlings for each of the six treatments = 360 plants). The data were subjected to an analysis of variance using the SAS GLM procedure, version 9.0 (Statistical Analysis System [SAS Institute], 2002) and Tukey’s multiple comparison test (P ≤ 0.05).
Results and discussion
Pathogenicity of Fusarium circinatum in Pinus greggii seedlings
Pathogenicity was determined based on the ability of the fungus F. circinatum strain SF5 to cause disease, which appeared 20 days after inoculation (dai). Robles, Gómez, Macas, Sánchez, and Torres-Gutiérrez (2014) inoculated babaco (Vasconcellea × heilbornii [V. M. Badillo] V. M. Badillo) plants with F. oxysporum Schltdl. through root wounds and observed symptoms at 15 days, while when they injected the inoculum into the substrate, symptoms took up to a month to appear.
The first manifestation of symptoms of the disease in P. greggii seedlings was recorded in the substrate made from pine bark (S3) (Figure 1A); however, it was similarly observed in all three substrates during the first week. The seedlings showed strangulation of the stem and wilting of the main shoot (Figure 1B); later, the needles turned yellowish and reddish. Likewise, Soria, Alonso, and Bettucci (2012) report that aerial symptoms in Pinus taeda L. seedlings do not appear until the pathogen reaches the neck area, resulting in wilting, loss of color (redness), needle drying and finally death. When extracting diseased seedlings, rotting of the main and secondary roots was observed; the roots had a light brown color. Wounds were not made in the P. greggii roots inoculated with F. circinatum and yet the disease occurred; this coincides with Peterson (2008), who points out that Fusarium spp. is capable of directly infecting the tissue, but differs with Soria et al. (2012), who state that the infection is associated with lesions or wounds in the bark or other tissues in the trees, because this fungus is unable to penetrate directly into the tissue.

Figure 1 Pathogenicity and effect of Fusarium circinatum in Pinus greggii seedlings planted in three substrates. A) Three- and five-month-old seedlings with substrate of pine bark, pine sawdust and peat moss (S3) at a 60:20:20 ratio. B) Symptoms with folding of the main shoot and decay. C) Seedlings with yellowish and reddish needles. D) Plant stem with mycelium and sporodochia of F. circinatum. E and F) Strains of the F. circinatum re-isolations.
Symptoms were most evident when the plant was four to six months old, as it showed folding of the main shoot, decay, and wilting with gradual brown changes until it became completely reddish, which is considered a symptom of death (Figure 1C). This coincides with the symptoms described by Herron et al. (2015), who point out that one-year-old seedlings show terminal bud fall and needle discoloration.
On the other hand, root rot was evident, as they were very brittle; this was seen in the inoculated plants of S1 and S3 when they were extracted from the cell. When the infection was severe, abundant mycelium was observed in the neck of the root and stem, and formation of white to orange sporodochia (Figure 1D). Out of a total of 480 plants inoculated with F. circinatum, 424 isolations of the associated fungi were made, giving 88 % positive re-isolations of the pathogen (Figures 1E and 1F); Koch’s postulates were verified with this identification.
The effect of F. circinatum on P. greggii showed a high mortality rate, coinciding with what was argued by García-Díaz et al. (2017) and Coutinho, Steenkamp, Mongwaketsi, Wilmont, and Wingfield (2007) when stating that F. circinatum is the most important pathogen in pine seedlings in different parts of the world. Also, Gordon, Swett, and Wingfield (2015) report that Fusarium species cause significant damage to coniferous seedlings and point to F. oxysporum as the most important agent causing hypocotyl rot in nursery seedlings.
Incidence of Fusarium circinatum in Pinus greggii seedlings
The results of this bioassay indicate that the mixture and the ratio at which the substrates were prepared had an effect on the incidence of disease caused by F. circinatum. The importance of this pathogen is shown in Figure 2, where it is observed that there was a direct effect on P. greggii seedlings in the first four weeks after inoculation of the substrate in the nursery.
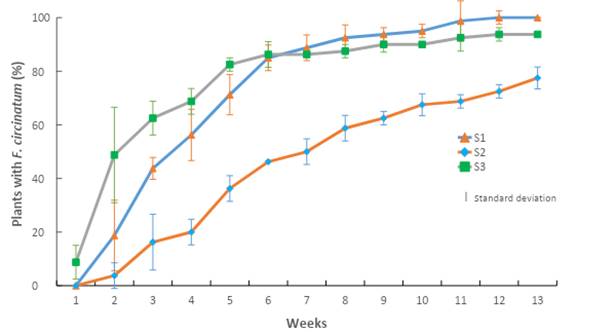
Figure 2 Accumulated incidence of Fusarium circinatum inoculated in Pinus greggii plants growing in three substrates: S1 = peat moss, perlite and vermiculite; S2 = pine sawdust, pine bark and peat moss; S3 = pine bark, pine sawdust and peat moss. All substrates were formulated at a 60:20:20 ratio.
Seedlings developed in sawdust-based S2 had a lower incidence of F. circinatum than those developed in S1 (based on peat moss) and S3 (based on pine bark), especially in the first four weeks of growth. In the following nine weeks, the percentage increased gradually until reaching an incidence of 94 % in S3 and 99 % in S1, while in S2 it was 78 %. The results in S1 are similar to those reported by Swett and Gordon (2015), who recorded an incidence of 97 % by inoculating F. circinatum, isolated from pine, in corn plants with substrate based on peat moss.
S2 reached an incidence of 78 %; this indicates that sawdust-based substrate may be an alternative to mitigate the incidence of F. circinatum disease in plant production nurseries. Additionally, costs could be reduced as indicated by Aguilera et al. (2016), who state that substrates mixed with pine sawdust and bark have been used successfully since 2003 in some nurseries in central Mexico.
Trichoderma harzianum as a biocontrol of Fusarium circinatum
Table 2 shows the results of the effect of T. harzianum on F. circinatum in P. greggii plants. Overall, in each substrate, treatments containing T. harzianum (T1, T5 and T9) were found to have lower incidence levels of diseased plants compared to treatments where only F. circinatum was applied (T2, T6 and T10); however, statistically, the incidence was significantly similar (P > 0.05). According to Table 2, of all treatments, T5 (S2+Fc+Th), sawdust-based and inoculated with T. harzianum, obtained the lowest incidence (66 %), while T2 (S1+Fc) without T. harzianum recorded the highest incidence of the disease (99 %). It appears that the use of S2 contributed to a reduction in the incidence of the disease.
Table 2 Effect of Trichodema harzianum (Th) as a biocontrol of Fusarium circinatum (Fc) at 30, 60 and 90 days after inoculation (dai) in Pinus greggii plants growing in three substrates.
| No. of treatment | Treatment | Incidence of diseased plants (%) | ||
|---|---|---|---|---|
| 30 dai | 60 dai | 90 dai | ||
| T1 | S1+Fc+Th | 21 ab | 69 ab | 86 a |
| T2 | S1+Fc | 56 bc | 88 ab | 99 a |
| T5 | S2+Fc+Th | 15 a | 43 a | 66 a |
| T6 | S2+Fc | 20 ab | 61 ab | 75 a |
| T9 | S3+Fc+Th | 11 a | 64 a | 86 a |
| T10 | S3+Fc | 69 c | 91 b | 93 a |
S1: peat moss, perlite and vermiculite; S2: pine sawdust, pine bark and peat moss; S3: pine bark, pine sawdust and peat moss; the ratio of materials in the three mixtures was 60:20:20. Average values with a different letter in a column are statistically different according to Tukey's test (P ≤ 0.05). Treatments T3, T4, T7, T8, T11 and T12 are not shown in the table because they were not inoculated with F. circinatum and did not show incidence.
It should be noted that only three applications of T. harzianum were made, so it is suggested to increase the number as recommended by Mitchell, Zwolinski, Jones, and Coutinho (2004), who state that applications with T. harzianum should continue for 180 days once the seedling emerges. It would also be important to determine whether T. harzianum is able to survive and remain viable in substrates to exert its antagonism; in this sense, Romero et al. (2008) studied the behavior of a strain of T. harzianum in two commercial-use substrates and found that composted bark-based substrate had a higher number of spores per gram of substrate.
Cubillos et al. (2011) evaluated six strains of the fungus F. solani (Mart.) Sacc. in the cultivation of yellow passion fruit (Passiflora edulis fo. flavicarpa O. Deg.) and pointed out that when T. harzianum is first applied there is a better biocontrol response (86 to 100 % of healthy plants) than by pre-infecting the seedlings with the pathogen; however, they also indicated a positive effect when F. solani was first applied (13 to 80 %).
Reglinski and Dick (2005) point out that suppression of damping-off disease was more effective when Trichoderma was added to the growth medium four days before inoculation of the pathogen and was ineffective when added four days after inoculation.
Mousseaux, Dumroese, James, Wenny, and Knudsen (1998) tested the use of T. harzianum for control of the pathogen F. oxysporum in seedlings of Pseudotsuga menziesii (Mirb.) Franco and obtained cumulative mortalities of 6 % in treatments with T. harzianum, 70 % with F. oxysporum, 80 % in the mixture of both fungi and 5 % in the control. This differs from the present study, as the treatments inoculated with T. harzianum and the control did not show mortality. On the other hand, Martínez-Álvarez, Alves-Santos, and Diez (2012) pointed out that T. harzianum, in dilutions of 107, 106 and 105, had a significant control effect against F. circinatum at the in vitro level, but in the P. radiata seedlings in the nursery they did not obtain satisfactory results.
Morphological indices of Pinus greggii seedlings inoculated with Trichoderma harzianum in three substrates
Table 3 presents the morphological characteristics and plant quality indices of P. greggii at six months of age. According to the information, the seedlings with the largest root neck diameter were obtained in the treatments with S1 and S3 (T3, T4, T11 and T12) with values from 3.03 mm to 3.17 mm, for which they would be considered medium-quality seedlings; to obtain a high quality, the diameter of the plant had to be greater than 4.00 mm (Prieto & Sáenz, 2011). The lowest values in diameter quality (P ≤ 0.05) were presented in T7 (S2+Th = 2.67 mm) and T8 (S2-C = 2.61 mm) containing substrate based on pine sawdust.
Table 3 Morphological characteristics and plant quality indices of Pinus greggii at six months of age, planted in three substrates (S) inoculated with Trichoderma harzianum (Th).
| Treatment | D (mm) | H (cm) | ADW (g) | RDW (g) | AP/RR | SI | DQI |
|---|---|---|---|---|---|---|---|
| T3 (S1+Th) | 3.11 a | 27.46 a | 1.35 a | 0.37 ab | 3.75 a | 8.90 a | 0.13 bc |
| T4 (S1+C) | 3.17 a | 28.14 a | 1.42 a | 0.43 a | 3.65 a | 8.95 a | 0.14 ab |
| T7 (S2+Th) | 2.67 b | 20.71 b | 0.96 c | 0.42 a | 2.35 c | 7.84 b | 0.13 bc |
| T8 (S2+C) | 2.61 b | 17.70 c | 0.78 d | 0.34 b | 2.33 c | 6.84 c | 0.12 c |
| T11 (S3+Th) | 3.03 a | 22.05 b | 1.07 bc | 0.42 a | 2.60 bc | 7.37 bc | 0.15 ab |
| T12 (S3+C) | 3.04 a | 21.39 b | 1.14 b | 0.43 a | 2.71 bc | 7.11 c | 0.16 a |
Average values in a column with a different letter are statistically different according to Tukey's test (P ≤ 0.05). S1: peat moss, perlite and vermiculite; S2: pine sawdust, pine bark and peat moss; S3: pine bark, pine sawdust and peat moss; the ratio of materials was 60:20:20. C = uninoculated, D = stem diameter; H = plant height; ADW = dry weight of the aerial part; RDW = root dry weight; AP/RR = aerial/root ratio (aerial dry weight/dry root weight); SI = slenderness index; DQI = Dickson quality index. Treatments T1, T2, T5, T6, T9, and T10 are not shown due to plant mortality by the pathogen.
Treatments with S1 (T3 = 27.46 cm and T4 = 28.14 cm), based on peat moss, promoted greater height in the seedlings; in fact, the height was greater than the 15 to 25 cm range reported by Sáenz, Villaseñor, Muñoz, Rueda, and Prieto (2010) to be considered as a high-quality plant. T8, corresponding to the substrate based on uninoculated pine sawdust (S2-Te), produced the lowest height value (17.70 cm). The results coincide with those of Castro-Garibay, Aldrete, López-Upton, and Ordaz-Chaparro (2018), who found that the peat moss substrate showed the best height and diameter growth in P. greggii var. australis Donahue, Jeffrey K. & López, A. R.
The values of height (except those obtained with S1) and diameter were below the established minimums (height of 25 to 30 cm and diameter ≥4 mm) in the criteria of the Mexican Standard for the Certification of Forest Nursery Operations NMX-AA-170-SCFI-2016 (Secretaría de Economía [SE], 2016) for P. greggii, probably due to the shadow effect of the greenhouse and the longer time required by the seedlings in the nursery. Villar (2003) states that the height of the aerial part and the diameter of the root neck, together with qualitative characteristics, are attributes considered in plant quality. Prieto and Sáenz (2011) report a stem diameter ≥4 mm and a height of 15 to 25 cm for pine species native to the Sierra Madre Occidental.
The aerial dry weight was also statistically higher (P ≤ 0.05) in S1 treatments (T3 = 1.35 g and T4 = 1.42 g), while the lowest was recorded in seedlings planted in S2 without inoculation (T8 = 0.78 g). In this same treatment (T8), the lowest root dry weight (0.34 g) was also recorded. The highest aerial part/root ratio was obtained in treatments with S1 (T3 = 3.75 g and T4 = 3.65 g). Aerial biomass was greater than underground biomass, which commonly occurs in several forest species.
S1 treatments showed higher slenderness indices (T3 = 8.90 and T4 = 8.95) than the rest of the treatments, which is not desirable, as they represent an imbalance in the plants. This influenced the Dickson quality index, which was less than 0.2 in all treatments as shown in Table 3.
In general, morphological standards did not differ significantly (P > 0.05) in the treatments where T. harzianum was applied with respect to the treatments where it was not applied, which coincides with the findings of Donoso et al. (2008). In contrast, Romero et al. (2008) found a positive effect on height, diameter, and root and aerial part weight when inoculated with T. harzianum. As for the substrates, the mixture based on peat moss as the main component (S1) had the best values for all evaluated variables, except for the slenderness index (Table 3). This coincides with what was found by Castro-Garibay et al. (2018) for the same species. The results show that more research is needed in our country in the forest area, since the effect of this antagonist has been more studied in the agricultural area (Dumroese et al., 2012).
Conclusions
Fusarium circinatum proved to be pathogenic in Pinus greggii seedlings in the three substrates evaluated. The sawdust-based substrate reduced the incidence of F. circinatum in the production of P. greggii seedlings in the nursery, mainly in the first four weeks after inoculation, unlike the substrate based on moss peat and pine bark that showed high incidence in this period. After 13 weeks, the application of T. harzianum and the sawdust-based substrate reduced the incidence rates of F. circinatum to 66 %; however, the incidence was similar when the antagonist was not inoculated. Fusarium circinatum is an economically important pathogen in forest nurseries and the combination of sawdust substrate and preventive application of the antagonist fungus T. harzianum is shown to be an option for its management.











 text in
text in 


