Introduction
The temperate forests of the Mexican Republic form a very important forest resource both for the extraction of wood and for the production of environmental services. These ecosystems host great biological diversity of flora and fauna, regulate the hydrological cycle, conserve the soil, and participate in carbon sequestration and storage, which contributes to regulating the climate and mitigating global warming (Segura-Warnholtz, 2014). However, these communities are affected by accelerated deforestation, habitat fragmentation and reduced forest density; moreover, many wooded areas are being displaced by bushes or are intended for other land use, either agricultural or livestock (Márquez-Linares, Jurado-Ybarra, & González-Elizondo, 2006).
In recent years, productive activity has intensified globally, which has contributed to the acceleration of climate change; an increase in temperature; changes in the intensity, spatial and temporal distribution of precipitation; and an increase in more extreme hydrometeorological phenomena (Secretaría de Medio Ambiente y Recursos Naturales [SEMARNAT], 2015). Temperate forest areas are considered the most sensitive to these changes (Villers-Ruiz & Trejo-Vázquez, 2004) and, according to vegetation change projections based on climate models (González-Elizondo, González-Elizondo, Tena-Flores, Ruacho-González, & López-Enríquez, 2012; Villers-Ruiz & Trejo-Vázquez, 2004), this is the type of vegetation that will lose more surface.
In recent decades, the observation of droughts in the national territory has gained relevance as a result of the discovery of the strong influence of the climate phenomenon El Niño Southern Oscillation (ENSO), which causes changes in the known climate cycles (Bitrán, 2001). Decreasing precipitation and increasing temperature are factors that weaken forests, making them susceptible to pests and diseases. On the other hand, pest outbreaks are also associated with tree stress due to factors such as competition, disease, insect defoliation, age and fire (Fetting et al., 2007). In Mexico, forest pests are considered one of the main causes of disturbance in temperate forests; currently there are records of about 70 species of insects and pathogens affecting trees (SEMARNAT, 2015). Climate change is modifying the biological cycle and distribution of forest pests in boreal coniferous forests and temperate regions, increasing the vulnerability of trees (Ayres & Lombardero, 2000).
In the United States of America and Canada (Allen, 2007; Berg, Henry, Fastie, De Volder, & Matsuoka, 2006; Breshears et al., 2005) and in European countries (Kozlov, 2008; Rouault et al., 2006), studies have been conducted on the indirect relationship of the effects of climate on the increase of bark beetle populations. Such studies are scarce in Mexico, a situation attributed to the lack of extensive climate information and the absence of historical records of pest outbreaks. Under such circumstances, this study proposes to generate a database of historical outbreaks of bark beetles in Mexico; analyze their historical relationship with droughts; and determine the influence of ENSO and the condition of the Palmer Drought Severity Index (PDSI) on the modulation of climate condition, for the historical incidence of pest outbreaks.
Materials and methods
Historical outbreaks of bark beetles
A literature review was conducted to gather information on historical records of outbreaks of bark beetles in northern, central, and southern Mexico, and in Guatemala and Honduras (Figure 1). The information was obtained from scientific and technical articles, scientific and technical brochures, scientific and technical notes, seminars and technical reports. Information from unofficial sources was discarded.
A database was created including the following variables: year and place of the outbreak, genus and species of bark beetles, species of conifer affected, and reference of the person who recorded the outbreak. Historical documents reporting significant droughts in Mexico were also consulted. The following variables were used as climate proxy:
1). A reconstruction of the precipitation for the southwest of the state of Chihuahua (winter-summer) based on growth rings of Pinus arizonica Engelm. including the period 1850-2015.
2). Dendroclimatic indices: for the north, a chronology of P. arizonica which comprises the period 1647-2015; for the center, a chronology of Pseudotsuga menziesii (Mirb.) Franco which comprises the period 1720-2013; and for the south, a chronology of Pinus patula Schiede ex Schltdl. & Cham. which comprises the period 1786-2015.
3). The winter index (December-February) NIÑO 3 Sea Surface Temperature (SST) (Cook, 2000).
4). Finally, the Palmer Drought Severity Index (PDSI) for June, July and August (JJA) for all Mexico (Stahle et al., 2016).
Data analysis
A first analysis consisted in relating, with a graphic, the historical database of outbreaks of bark beetles and the annual climate variability that characterizes the north of Mexico. Because the observed records are not extensive, winter-summer rainfall reconstruction was used for the southwestern state of Chihuahua. This analysis allowed us to see a first relationship between the outbreaks of bark beetles and droughts.
In a second phase, the relationship between the dates of the outbreaks of bark beetles per region (north, center and south of the country, this last region includes the data from Guatemala and Honduras) and the climate variability that characterizes each of the regions was analyzed statistically with the routine SEA (Superpose Epoch Analysis) of program FHX2 version 3.2 (Grissino-Mayer, 2001).
Each of the climate variables (dendroclimatic indices, NIÑO 3 index and PDSI) was analyzed separately with the bark beetle outbreaks, a procedure that consisted in comparing the climate conditions during the year of the outbreak, five years before and two years after the outbreak. To assess the statistical significance of the SEA analysis, confidence intervals (95, 99 and 99.9 %) were estimated using the "bootstrapped" distribution of climate data with 1 000 replications.
Finally, drought maps (PDSI) were generated to analyze the climate conditions prior to and during the outbreak, using The Mexican Drought Atlas (MXDA) (Stahle et al., 2016).
Results and discussion
Historical records of bark beetle outbreaks
It was possible to compile a database of 106 records of bark beetle outbreaks in 15 states of Mexico, distributed in the north, center and south of the country during the period 1903-2015 (Figure 1; Appendix 1), where Dendroctonus mexicanus Hopkins, Dendroctonus frontalis Zimmermann and Dendroctonus adjunctus Blandford were the most frequent species. Guatemala recorded 16 events during the period 1895-2013, D. frontalis and D. adjunctus were the most important species; and Honduras recorded 15 outbreaks of D. frontalis during the period 1962-2015 (Figure 1; Appendix 1).

Figure 1 Geographical distribution of bark beetle outbreaks recorded in the last 120 years (1895-2015).
The history of bark beetles outbreak in Mexico is 113 years (1903-2015) (Figure 2b). The lack of information on bark beetle infestations prior to 1900 and scarce records prior to the 1970s are attributed to the fact that, for these periods, the true magnitude, location, and characterization of the pest were not yet defined.
Relationship between pest outbreaks and droughts
Figure 2 shows with a graphic the relationship between bark beetle outbreaks and droughts in the study area. Outbreaks have been historically recorded in years with below-average precipitation (550 mm) (Figure 2a). During the last 40 years (1972-2012), the number of bark beetle outbreaks increased throughout Mexico, which is associated with very severe droughts. In the 1990s, the number of outbreaks increased from 1996, with 1999 being the year with the greatest impact. In the first decade of the 21st century, the presence of bark beetle outbreaks worsened in 2000, 2001, 2002, 2009, 2011 and 2012 (Figure 2b).

Figure 2 Record of bark beetle outbreaks in Mexico, Guatemala and Honduras. (a) Reconstructed winter-summer precipitation for southwestern Chihuahua; the gray bottom line represents annual variability, the black line is a flexible 10-year scale curve for analyzing dry and wet events, and the dotted horizontal line indicates regional average precipitation. Historical outbreaks of bark beetles. Horizontal lines represent states and black vertical bars indicate years of outbreaks; as the line becomes continuous it indicates outbreaks on a larger geographic scale. Vertical red lines show the relationship between the frequency of pest outbreaks and below-average precipitation (droughts).
Each of the periods reported with the highest incidence of outbreaks, from the 1970s to the first decade of the 21st century, synchronize with droughts documented in dendroclimatic reconstructions in northern and central Mexico (Cerano-Paredes et al., 2009; Cleaveland, Stahle, Therrell, Villanueva, & Burns, 2003; Therrell, Stahle, Cleaveland, & Villanueva-Díaz, 2002; Villanueva, Fulé, Cerano, Estrada, & Sánchez, 2009).
One of the periods with intense drought throughout the country is that of the mid-twentieth century (Cerano-Paredes, Villanueva-Díaz, Valdez-Cepeda, Méndez-González, & Constante-García, 2011; Stahle et al., 2009), as an extreme drought occurred in the 1950s that impacted Mexico and much of the southwestern United States of America (Florescano, 1980); however, no reports were found for this period that indicate pest outbreaks in Mexico (Figure 2). This lack of information is attributed to the fact that, in 1950, the importance of forest pests was still not properly documented, either due to lack of vigilance, lack of knowledge or simply because large forest stands were not yet affected; infestations by bark beetles began to attract attention in the 1960s (Islas-Salas, 1980).
During the 1970s, reports of forests affected by bark beetles increased, a situation that prompted addressing the problem and proceeding with the study and control of the pest (Islas-Salas, 1980). This is attributed to the fact that, from 1970 to the beginning of the 1980s, intense droughts were documented for Mexico in which the droughts recorded in 1971, 1972, 1974, 1975, 1976 and 1977 stand out. The droughts of 1974 and 1975 were recorded throughout the Americas, while the drought of 1977 is classified as extremely severe (Florescano, 1980). Historical reconstructions of precipitation variability throughout the country have reported these same periods with significant decreases in precipitation (Cardoza-Martínez et al., 2014; Cerano-Paredes et al., 2009, 2014; Cleaveland et al., 2003; Stahle et al., 2016; Villanueva et al., 2009).
For the last decade of the 20th century (1994 to 2000) and the years 2001 to 2003, 2006, 2009, 2011 and 2012 of the 21st century, both the observed records of weather stations (Instituto Mexicano de Tecnología del Agua [IMTA], 2009) and the dendroclimatic reconstructions for northern and central Mexico (Cardoza-Martínez et al., 2014; Cerano-Paredes et al., 2009, 2014; Villanueva et al., 2009) detected high-intensity droughts. This is in accordance with the records of outbreaks of bark beetles reported in recent decades in coniferous forests of North and Central America; high temperatures combined with drought, together with factors such as logging and forest fires, have contributed to generating a higher incidence of bark beetle outbreaks, whose damage has increased, particularly since 2000 (Moore & Allard, 2009).
Pest outbreaks-climate variability
The SEA between bark beetle outbreaks and climate indices indicates that documented pest outbreaks in the regions of Mexico, Guatemala, and Honduras have historically been recorded during significantly dry years (P < 0.05) and with index values below average (dry) from five, four, and two years prior to the outbreak to the north, center, and south of the country, respectively (Figure 3).
Below-average climate conditions during previous years cause trees to weaken, making them susceptible to attack. The direct effects of warm temperatures on the reproductive capacity of bark beetles generate the ideal conditions for an outbreak (Bentz et al., 2010; Brunelle, Rehfeldt, Bentz, & Munson, 2008). The results obtained coincide with what has been reported by several authors (Breshears et al., 2005; Islas-Salas, 1980; Negrón, McMillin, Anhold, & Coulson, 2009; Raffa, Aukema, Erbilgin, Klepzig, & Wallin, 2005; Williams et al., 2013), who point out that drought events are an important factor for an imbalance; in the particular case of bark beetles, the population increases under these conditions. Also Mattson and Haack (1987) mention that droughts lead to bark beetle outbreaks by creating a more favorable thermal environment; plants stressed by drought are more attractive and physiologically susceptible to these insects, by negatively influencing resin production mechanisms in conifers, their main means of defense. The probability of a successful attack increases under drought conditions, also because it favors the reduction of carbon assimilation and water transport, affecting the capacity of the tree to produce non-structural carbohydrates and mobilize secondary metabolites for the production of defenses (Safranyik & Carroll, 2006).
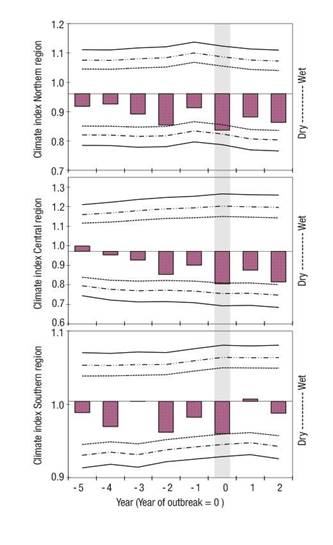
Figure 3 Relationship between historical pest outbreaks and regional climate variability (north, center and south) in Mexico, through the SEA (Superpose Epoch Analysis) of the program FHX2. The southern region also includes data from Guatemala and Honduras. The year of the outbreak is indicated by 0, climate conditions five years before the outbreak (negative values) and two years after the outbreak (positive values). The top and bottom three lines represent the 95 %, 99 % and 99.9 % confidence intervals. The shaded bar indicates the climate index values (statistically significant drought) during the years of bark beetle outbreaks in the study area.
Pest outbreaks-ENSO-PDSI
Figure 4 shows the SEA between the bark beetle outbreaks and the ENSO and PDSI climate indices. Outbreaks in Mexico, Guatemala and Honduras have historically been recorded in years with non-significant positive indices (P > 0.05) of NIÑO 3 and PDSI, after highly significant drought conditions (P < 0.01) in the previous year and with negative indices of NIÑO 3 and PDSI.

Figure 4 SEA (Superpose Epoch Analysis) between the historical outbreaks of bark beetles and el NIÑO 3 SST (Sea Surface Temperature) and PDSI (Palmer Drought Severity Index) for the months of June, July and August (JJA). The shaded bar shows the statistically significant drought conditions (negative indices) one year prior (-1) to the bark beetle outbreaks. The dotted line indicates the year of the outbreak under non-significant positive index conditions.
Mexico is highly vulnerable to extreme climate events, especially in mid-latitudes and subtropical areas (Martínez-Austria & Patiño-Gómez, 2012). The climate phenomenon known as ENSO is one of the most important circulatory atmospheric patterns that affect the climate variability of the country and is directly related to the occurrence of droughts (Magaña, Vásquez, Pérez, & Pérez, 2003). Since the 21st century, bark beetle outbreaks have increased in frequency, severity and extent (Raffa et al., 2008). The climate variability in the last decades has been pointed out as one of the causes of the recent increase in the outbreaks of bark beetles in the United States and Canada (Hicke, Logan, Powell, & Ojima, 2006; Thomson, 2009). In Honduras, the degree of aggressiveness and intensity of bark beetles in recent years has been influenced by the presence of ENSO, which has produced prolonged drought events (Comisionado Nacional de los Derechos Humanos [CONADEH], 2016).
Outbreaks of many bark beetle species are occurring simultaneously throughout western North America. The variety of factors involved is diverse, with drought-induced changes in host susceptibility (Breshears et al., 2005), management practices (Keane, 2001), and the direct effects of warm temperatures on beetle reproductive capacity (Berg et al., 2006).
Unexpected changes in climate conditions have a direct influence on bark beetle outbreaks (Brunelle et al., 2008). Drought conditions for one or two consecutive years lead to a state of physiological stress on trees, making them more susceptible to bark beetle attack and causing epidemic outbreaks on a wide geographic scale (Figures 4 and 5). A documented example is El Niño of 1998 which represents one of the driest years of the 1990s, in which a large number of fires were recorded in the forests of Mexico. The report of fires that occurred in 1998 exceeded the forecasts of the institutions in charge of fire prevention and firefighting in the national territory (Bitrán, 2001). Both variables, drought and fires caused stress in plants, an attractive and physiologically condition for bark beetles (Mattson & Haack, 1987). This disturbance favored the development of a large number of bark beetle outbreaks in 1999, from the north to the south of Mexico (Sánchez-Salas & Torres-Espinosa, 2007). Like this event, in 1895 and 1972 a similar behavior was determined; in other words, extreme drought conditions modulated by ENSO (negative values of PDSI), previous to the important outbreaks of bark beetles (Figure 5).

Figure 5 Drought maps created with the Mexican Drought Atlas (Stahle et al., 2016) available at http://drought.memphis.edu/MXDA/. The values on the -6 scale indicate the most severe drought condition and 6 the most humid condition. PDSI: Palmer Drought Severity Index for June, July and August (JJA).
In Mexico, it has been documented that the growth of conifers is regulated and limited by water availability (Cardoza-Martínez et al., 2014; Cerano-Paredes et al., 2014; Chávez-Gándara et al., 2017; Villanueva et al., 2009); therefore, when important decreases in rainfall are recorded, trees weaken and are more susceptible to bark beetle attack. Regarding these results and the report of the Intergovernmental Panel on Climate Change (IPCC, 2014) that projects greater drought as a consequence of the decrease in precipitation and increase in temperature, periods of drought and greater risk for forests can be inferred in the face of an increase in bark beetle outbreaks.
Conclusions
Climate is a factor that modulates the increase in bark beetle populations. In the face of climatic scenarios that project a condition of greater drought, it is evident that pest outbreaks can be magnified. Because of this, it is necessary to take appropriate precautions in forests that are susceptible to demographic pressure and those that have very dense conditions, mainly when there are one to two consecutive years of droughts. Likewise, it is important to monitor forests after fires, since there is a direct relationship with the presence of pest outbreaks after a fire attributed to the physiological stress to which trees are subjected. Understanding ENSO's influence on inter-annual and multiannual climate variability and determining drought indices can contribute to establishing preventive measures to minimize the impact of bark beetles on Mexican forests.











 texto en
texto en 


