Introduction
The Intergovernmental Panel on Climate Change (IPCC) has predicted greater variability in the component processes of climate change in North America, where a decrease in precipitation and increased CO2 concentration and temperature are predicted (Ford et al., 2016).
Mexico is a country with a great diversity of climates and ecosystems due to its geographical position; however, arid and semi-arid climates predominate, covering almost 50 % of its area (Garatuza-Payan, Argentel-Martinez, Yepez, & Arredondo, 2018). In semi-arid climates, such as those in southern Sonora, the development of vegetation, soil microbial activity and the ecosystem are hampered by the negative incidence of abiotic factors such as low or no rainfall, soil salinity and a significant temperature increase (Lares-Orozco, Robles, Yépez, & Handler, 2016). Drought and salinity affect the physiological performance of plants after alteration of their optimal development, modifying their water relations, mainly their transpiration rate, photosynthesis-respiration balance and water-use efficiency (Azcón-Bieto & Talón, 2008; Cary & Pittermann, 2018).
Some studies carried out in northwestern Mexico indicate that not all species will tolerate climate change (Cavazos & Arriaga, 2012). Therefore, monitoring the response of species in these ecosystems could be an important step in establishing reforestation programs and thus reducing the evaporation surface, promoting better gas exchange in the ecosystem and, finally, increasing its functionality.
The mesquite (Prosopis laevigata [Humb. & Bonpl. ex Willd.] M. C. Johnst.) is a tree species that is a fundamental part of Mexico’s semi-arid ecosystems and that in certain cases becomes dominant, constituting forests (Manjarrez, 2017; Rodríguez et al., 2014). The species is of great economic, ecological and social importance in non-agricultural regions of northern Sinaloa and southern Sonora. One of the most important products is wood, highly valued for its strength and quality for charcoal production (Galindo-Soto, Vargas-Larreta, Hernández, & Cruz-Cobos, 2017). In northwestern Mexico, the mesquite has served not only as a source of food for cattle (Martín, Flores, Moreno, Retes, & Amarillas, 2016) and, recently, for human consumption (Boeri, Piñuel, Sharry, & Barrio, 2017), but also as an economic and medicinal heritage of indigenous communities such as the Yaqui and Mayo tribes (Herrera, Moreno, Villares, González, & Belmonte, 2017); it has also shown high survival in the competition for wood for charcoal (Hernández-Herrera, Valenzuela-Núñez, Flores-Hernández, & Ríos-Saucedo, 2014). Although there is information on the wood yield, diversity and distribution of mesquite in ecosystems (García et al., 2017), little has been studied about the physiological and biochemical parameters related to its water regime and carbonated nutrition that demonstrate tolerance and adaptation to drought and salinity conditions. The results of the present research show the response of P. laevigata to salinity and drought conditions, through the evaluation of water and osmotic potentials, photosynthesis, transpiration and water-use efficiency in two semi-arid ecosystems of southern Sonora.
Materials and methods
The research was conducted during the dry season (December 2017 - April 2018) in the southern region of the state of Sonora, Mexico, in two experimental sites: Eco Camping, representative of a semi-arid ecosystem (27° 22' 07" N and 109° 49' 12.874" W), and in block 1217 of the village of Bahía de Lobos (27° 21' 06" N and 110° 27' 14" W), representative of soils affected by salinity. These sites constituted the treatments in the research: T1) salinity condition and T2) drought condition. The variables were measured in triplicate during three alternate days in the second half of April 2018.
Climate variables
The average monthly temperature remained between 17 and 24 °C (average 18.6 °C in Eco Camping and 16.71 °C in Bahía de Lobos). Monthly precipitation was less than 0.2 mm and relative humidity ranged from 50 to 67 % at both sites (Figure 1).
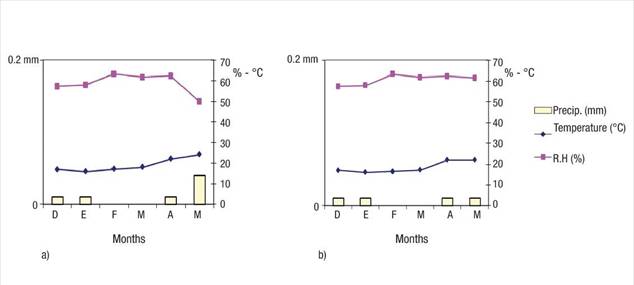
Figure 1 Climate variables (temperature, precipitation and relative humidity) during the experimental period (December 2017 - April 2018) at Eco Camping (a) and Bahía de Lobos (b) in southern Sonora, Mexico. Source: Red de Estaciones Meteorológicas Automáticas de Sonora (REMAS, 2018).
Soil condition of experimental sites
Samples were taken from the soils of both sites at depths of 0 to 70 cm. Some variables such as moisture percentage, organic matter percentage and cationic content were determined (Table 1) in the Soil Testing Laboratory of the Instituto Tecnológico de Sonora, using the methodologies set out in standard NMX-AA-132-SCFI-2016 (Secretaría de Economía, 2016) for such purposes. Both sites have Fluvisol soils (Barriga, Olguin, Almanza, Espinoza, & Coronado, 2018), a classification that correlates with the Soil Taxonomy methodology (Bockheim, Gennadiyev, Hartemink, & Brevik, 2014).
Table 1 Physical and chemical characteristics of semi-arid (Eco Camping) and saline (Bahía de Lobos) soils with presence of mesquite (Prosopis laevigata) in southern Sonora, Mexico.
| Sites | Texture | Moisture (%) | OM (%) | pH | EC (dS·m-1) | Cations (meq·L-1) | Anions (meq·L-1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Na | Ca | Mg | SO4 | CO3 | HCO3 | Cl | ||||||
| Eco Camping | Sandy | 66 | 0.9 | 7.2 | 0.96 | 1.62 | 0.7 | 8.1 | 3.50 | 2.36 | 1.22 | 1.14 | 1.1 |
| Bahía de Lobos | Sandy | 76 | 0.4 | 6.1 | 2.17 | 1.13 | 9.7 | 7.8 | 5.23 | 4.59 | 0.85 | 3.34 | 7.5 |
OM = Organic matter, EC = Electrical conductivity.
Variables evaluated
Three pure 5 x 5 m stands were randomly established at each experimental site, in which three plants of the species P. laevigata were identified for a total of nine replicates. The physiological evaluation was done on plants with similar morphological traits (height of 1.5 m and stem diameter of 0.15 m measured at 1.3 m).
Water potential
Water potential was determined in three samples of secondary roots at a depth of 40 cm, in three stem fragments (approximately 1 cm in diameter) from the upper third of their branches, and in three green leaves exposed to full solar radiation. Each sample was placed in the sample holder of the Scholander pressure chamber (PWP-C04) (Scholander, Bradstreet, Hemmingsen, & Hammel, 1965) at 10:30 am, before the temporary wilting point. The pressure (<2 MPa) was supplied until liquid appeared at the end of the sample and on the petiole of the leaves.
Osmotic potential
Osmotic potential, in saturated weight condition, was determined in samples adjacent to the three organs. For handling, the samples were quickly placed in Ziploc® double-zipper bags and sealed. In the laboratory, the samples were placed in Petri dishes and rehydrated with distilled water, then put back into Ziploc double-zipper bags and kept between 6 and 8 °C for 12 hours. The samples were then wrapped in aluminum foil and frozen in liquid nitrogen at -80 °C. Finally, the samples were thawed at room temperature and centrifuged at 3 000 rpm for 3 min to obtain the cell juice from the leaves. The osmotic potential of the leaves was determined in triplicate with a vapor pressure osmometer (Vapro 5520, Wescor Inc., EUA), from 100-mL aliquots.
Gas exchange
In each ecosystem, gas exchange was measured on leaves exposed to full radiation, using an LI-6400 system (Portable Photosynthesis System, Li-Cor, Lincoln, USA) equipped with a standard 2 x 3 cm camera with natural light source. The measurements were made on sunny days from 10:00 to 12:00 a.m., with a luminous intensity (photosynthetically active radiation) of 1 500 μmol· m2·s-1 to determine the maximum photosynthesis (A(max)) and with a CO2 concentration of 400 μmol·mol-1 with a constant flow of 500 μmol·s-1 (Carmona, Trejo, Esquivel, Arreola, & Flores, 2007). The most important variables were photosynthesis (A(max) in μmol CO2·m2·s-1) and transpiration (E in μmol H2O·m2·s-1). Water-use efficiency was determined by the A(max)/E ratio. Every 10 s, the equipment recorded one replicate of each sampled leaf for a total of nine replicates per plant. A total of 27 plants were sampled for these variables.
Statistical analysis
Data were subjected to the theoretical assumptions of Bartlett’s normality test and Kolmogorov-Smirnov’s variance homogeneity, and the standard deviation and error of the mean of the evaluated variables were determined. The water and osmotic potential variables were compared by means of a two-way ANOVA, based on a fixed-effects linear model without interaction between factors (Fisher, 1937), taking the measurement organs (three levels: roots, stems and leaves) as sources of variation and the sites as treatments (two levels: salinity and drought). When differences existed, the means were compared with Tukey's test (P < 0.05 and P < 0.01) (Tukey, 1960). The remaining variables (photosynthesis, transpiration and water-use efficiency) were compared through a hypothesis test, following a theoretical distribution of probabilities for continuous Student’s t quantitative variables (P < 0.05 and P < 0.01) (Gosset, 1917). The professional statistical package STATISTICA version 10.1 for Windows (StatSoft, Inc., 2010) was used in all analyses.
Results and discussion
Water potential
Water potential showed significant differences (P = 0.0037) between the evaluated ecosystems, showing a greater decrease in the salinity-affected ecosystem plants. The smallest difference in water potential between salinity and drought conditions was found in the stem; even so, it was significant (P = 0.0046) (Figure 2). In both conditions, the decreased potential in the leaves was significant (P = 0.0041), showing a potential gradient from the root to ensure a good water state in the plants and to maintain transpiration. The variability found in the measurement of water potential was attributed in greater percentage to the effect of ecosystems (R2 = 0.78), while the effect of the organ factor only represented 11 % of variability (R2 = 0.11), without interaction among these variation factors.

Figure 2 Water potential in Prosopis laevigata roots, stems and leaves in ecosystems affected by salinity (Bahía de Lobos) and drought (Eco Camping) in southern Sonora, Mexico. SD: standard deviation of the mean; R2: Unadjusted coefficients of determination for ecosystem and organ variation sources.
The decrease in water potential ensures the absorption of sufficient water and the maintenance of adequate transpiration (Lovelock, Krauss, Osland, Reef, & Ball, 2016). At root level, when water potential is not reduced as a function of osmotic potential, the hydraulic conductivity is modified and the water absorption towards the leaves is favored, avoiding cell damage (Saradadevi, Bramley, Palta, Edwards, & Siddique, 2016). This mechanism has been observed in stress conditions due to salinity (Sadras, Villalobos, Orgaz, & Fereres, 2016) and drought (Saxena, Prasad, Parashar, & Rathi., 2016). Plants show a significant increase in osmotically active compounds, mainly of organic origin, to rcompensate for the physiological drought created by water loss in transpiration (Tasmina, Khan, Karim, Akter, & Islam, 2017).
Osmotic potential
Osmotic potential also showed highly significant differences (P = 0.00012) between conditions and among organs. These values also decreased at leaf level with respect to root level. The greatest decrease was obtained in the saline ecosystem (Figure 3). Although there was significant interaction among the variation sources, it only contributed 17 % to the total variability of osmotic potential. On the other hand, due to the ecosystem effect, the highest percentage of variability (R2 = 0. 57) was obtained, while 29 % was explained by the organ factor effect (R2 = 0.25). This result reaffirms the ability of the species to maintain good water status as a function of the decreased osmotic potential in its organs, an aspect that is more evident in the saline ecosystem, either by the accumulation of ions or by osmotically active compounds (Blum, 2017).
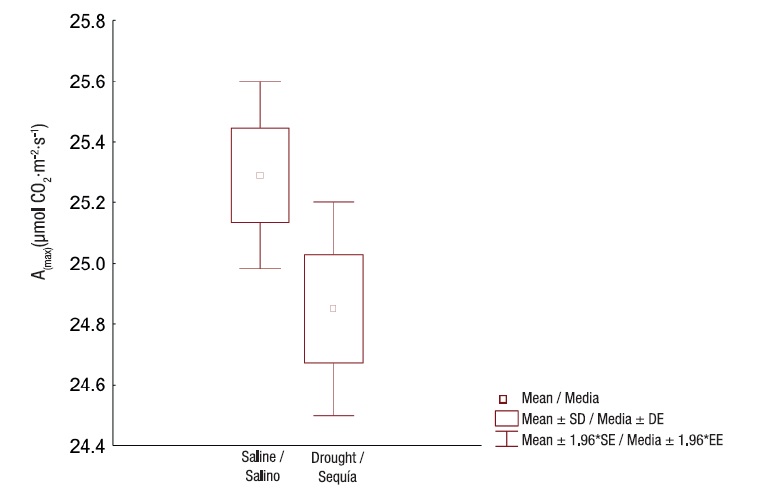
Figure 3 Osmotic potential in Prosopis laevigata roots, stems and leaves in ecosystems affected by salinity (Bahía de Lobos) and drought (Eco Camping) in southern Sonora, Mexico. SD: standard deviation of the mean; R2: unadjusted coefficients of determination for ecosystem and organ variation sources and their interaction.
In general, the ability to decrease the osmotic potential in plants has been an important variable in the differential selection programs of species with tolerances to stress conditions, to mitigate the effects of physiological drought generated under stress conditions such as salinity (Rosabal et al., 2014). This drought is not due to the absence of water but to the difficulty of it being absorbed by the plants, due to the low osmotic potential of the element with respect to that of the plant (Cary & Pittermann, 2018).
The decreased water potential allows P. laevigata to maintain an adequate water balance under stress conditions due to salinity (Blum, 2017) and drought, to support transpiration and to avoid the physiological drought generally associated with saline stress (Miyazawa et al., 2016). It has been determined that, under salinity conditions, some species avoid diminishing their osmotic potential at the expense of the inclusion of cations and anions that may promote ionic toxicity and nutritional interference, such as Na+ and Cl- by K+ (Harfouche, Meilan, & Altman, 2014). However, some species achieve inclusion of ions and compartmentalization to ensure a low osmotic potential and, consequently, a low water one too, as well as to absorb water in soil salinity conditions (Sachan & Jain, 2017).
Certain species, especially coastal ones, manage to carry out osmotic adjustment (OA) to ensure the water regime. OA is a physiological trait generally associated with drought stress and has been proposed as a selection variable in breeding programs for warm climates, due to the great ability that plants develop to absorb water strongly retained in soil capillaries (O´Brien et al., 2017). In tree species, the introduction of this variable in the differential models of genetic improvement for stress in semi-arid and coastal ecosystems has been very successful. In these ecosystems, the availability of water is not due to its absence but to the difficulty of absorbing it, due to the low water potential of the existing water in the subsoil (Chhabra, 2017).
The favorable response of plants to physiological drought, due to their osmotic adjustment ability, is an additive-type trait (Zhu et al., 2018) that is easy to inherit, so it can be introduced into breeding programs to obtain tolerant materials (Gehring, Sthultz, Flores-Rentería, Whipple, & Whitham, 2017).
Photosynthesis, transpiration and water-use efficiency
Photosynthetic activity showed no significant differences (P = 0.039) between the evaluated sites; values exceeded 23 µmol·m2·s-1 (Figure 4). Such values are considered sufficient to maintain an adequate carbon balance, a sign of carboxylation efficiency (Santos et al., 2018).

Figure 4 Maximum photosynthesis in Prosopis laevigata species in ecosystems affected by salinity (Bahía de Lobos) and drought (Eco Camping) in southern Sonora, Mexico. SD: standard deviation of the mean, SE: standard error of the mean.
Research on the effects of abiotic stress on the photosynthetic activity of forest species is not sufficient, and existing research diverges in the results, even under the same stress condition (Asao & Ryan, 2015; Scafaro et al., 2017); in addition, soils with degradation problems are usually used for the establishment of forest species, because they are only established for timber production and not for research related to abiotic stress in forest physiology (Ayala-Orozco et al., 2018). Drought and salinity stress can accelerate senescence in photosynthetic organs, causing reductions in chlorophyll content and functionality. There is evidence that the concentration of chlorophyll pigments a and b can be reduced differentially by the effect of heat (Tsai, Harding, & Cooke, 2018), indicating that stressful conditions reduce photosynthetic capacity in general.
The ability of some species to maintain a high chlorophyll content (stay-green) and their functionality in the face of drought or salinity stress is a trait that could help maintain the supply of carbon skeletons needed for the synthesis of protective osmolytes. These allow the osmotic and water potential to be kept low enough to ensure the plant’s water regime (Jafarnia, Akbarinia, Hosseinpour, Modarres, & Salami, 2018).
Transpiration, on the other hand, was significantly different (P = 0.0003) between the evaluated sites, with a lower value obtained in the salinity-affected ecosystem (Figure 5). This result shows that salinity affects respiration to a greater extent than drought.
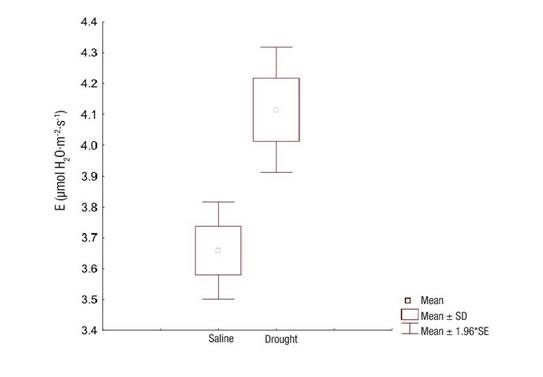
Figure 5 Transpiration (E) of Prosopis laevigata in ecosystems affected by salinity (Bahía de Lobos) and drought (Eco Camping) in southern Sonora, Mexico. SD: standard deviation of the mean, SE: standard error of the mean.
With respect to water use, efficiency was higher in the salinity-affected ecosystem, mainly due to the lower existing transpiration (Figure 6). Evaluation of water-use efficiency has been proposed as one of the most accurate indicators for the selection of crop genotypes in semi-arid areas with deficit irrigation regimes (Killi et al., 2018).
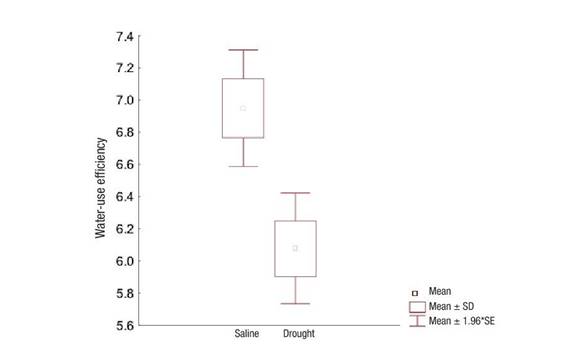
Figure 6 Water-use efficiency in Prosopis laevigata in ecosystems affected by salinity (Bahía de Lobos) and drought (Eco Camping) in southern Sonora, Mexico. SD: Standard deviation of the mean, SE: Standard error of the mean.
There are several elements that verify water-use efficiency at the crop and ecosystem level, which are difficult to control; however, even at leaf scale, efficiency is difficult to measure in the field, as it requires gas exchange measurement equipment. One of the most practical alternatives has been the photosynthesis/transpiration (A/E) ratio (Chebbi et al., 2018), hence the importance of current measurement. Water-use efficiency is also regulated by water potential, as a more direct measure of the degree of free energy of water; novel techniques have been recently developed for the determination, due to its importance in the functionality of semi-arid ecosystems (Weiwei et al., 2018).
Social contribution of Prosopis laevigata and the study of water relations
Prosopis laevigata has been part of the cultural and economic heritage of many generations, mainly indigenous, and has contributed to the increase in species biodiversity and ecosystem functionality (Miranda & Alejo, 2017). The importance of studying the mesquite lies in the need to implement fast and precise reforestation programs based on assessments of the plant’s tolerance to prevailing stress conditions in the semi-arid region of Mexico, particularly in southern Sonora.
Salinity and drought conditions modify the water status of the mesquite, due to the low water potential of soils that hinder the absorption of water and the normal metabolism of plants. The study of the water regime will allow multi-criteria decisions to be made to regionalize the establishment of the species and ensure good development and productivity.
P. laevigata trees are beneficial and greatly valued because they require low investments for their establishment, maintenance and productivity (Gómez, Torres, & Suárez, 2017). However, no matter how minimal the investment, it often exceeds the social, financial, legal and ecological capabilities of rural communities. For this reason, government authorities, local residents, scientists and technicians must take into account the appropriate information for the treatment of the species and thereby avoid its disappearance from ecosystems due to misuse and management, and due to the economic and financial issues of the community itself (Osuna & Meza, 2003). The silvopastoral system and forestry require greater attention and regulation of biosecurity, in order to prevent some biological risks and thus prevent this species from continuing to be called an invasive tree (Rodríguez et al., 2014). In addition to the mesquite's potential to combat desertification, the species is also a food source for both livestock and residents in rural communities, as well as a source of firewood and charcoal (García et al., 2017); the sustainable control of the species, its biosecurity and the prevention of its irrational exploitation will allow these benefits to be exploited. On the other hand, the exploitation of the products that can be obtained from mesquite such as wood, pods and honey represent the generation of an economic income that also benefits the communities (López-Franco, Goycoolea, Valdez, & Calderón, 2006).
Conclusions
The salinity condition led to a greater decrease in the water and osmotic potentials of Prosopis laevigata compared to the drought condition. This species, in both ecosystems, maintained a potential gradient and thereby evidenced the activation of mechanisms of tolerance to these types of stress. Photosynthetic activity did not vary due to salinity and drought conditions; however, the presence of salts caused a decrease in transpiration and, consequently, greater water-use efficiency. This study demonstrated the ability of P. laevigata to tolerate stressful salinity and drought conditions in non-agricultural ecosystems in southern Sonora, where the species predominates.











 texto en
texto en 


