Introduction
Mexico is considered as the secondary center of diversity of pinyon pines; despite this, it contains the highest richness of the Cembroides subsection, since it is home to 12 of the 16 existing taxa, of which 10 are considered endemic (Gernandt & Pérez-de la Rosa, 2014; Sánchez, 2008).
Pinyon pines are the dominant species in the plant communities where they grow, harboring great floristic diversity that is reflected in the variety of plant associations of unique physiognomy. This structural complexity is due to the fact that pinyon pines have wide latitudinal and elevational distribution, occupying different climatic and edaphic regimes, due to the interaction of environmental and ecological factors (Rzedowski, 2006; Sánchez-González, Álvarez-Zúñiga, & López-Mata, 2016). In turn, the communities of which pinyon pines are part are small, isolated forests, characterized as ecotones hosting xerophytic and temperate species (Farjon & Styles, 1997). To understand the complex structure of pinyon pine forests in different regions of the country, floristic and physiognomic characterization and the use of multivariate analysis techniques have been used (Chavoya, Granados, Granados, & Esparza, 2016; Granados, Granados, & Sánchez, 2015; Romero, Luna, & García, 2014). The use of these methods has allowed detecting the environmental variables that determine the floristic composition and structure of pinyon pine forests.
Pinyon pine forests are one of the most productive ecosystems in arid areas. These forests provide multiple environmental services such as soil conservation and production, water retention and carbon sequestration; in addition, they are fundamental in the local economy (Reyes-Carrera, Méndez-González, Nájera-Luna, & Cerano-Paredes, 2013) for the production of fuels, pine nuts, ornamental plants, alcoholic beverages (mezcal, pulque and sotol) and medicinal treatments. However, these ecosystems are the least studied by the forestry sector since they are considered a non-timber resource, so pinyon pine forests are highly vulnerable due to a lack of adequate management (Hernández, Islas, & Guerra, 2011). This highlights the need to generate information for the sustainable use of pinyon pine forests, which requires knowing the environmental factors that influence the composition and structure of the vegetation. The study of these ecosystems serves as the basis for the development of plans for the management and use of forest resources; in addition, it promotes the conservation of pinyon pines, as well as the associated flora and fauna, in order to maintain the goods and services that these forests provide (Granados, Hernández, & López, 2012).
There are several sites in the state of Zacatecas with pinyon pine populations; however, there is no adequate quantitative record on the floristic richness and ecology of these areas. Therefore, the objective of this study was to describe the floristic attributes that distinguish communities dominated by pinyon pines (characterization), to define units based on their floristic composition and structure (classification) and determine the influence of edaphic factors (ordination).
Materials and methods
Study area
Four pinyon pine forest communities were analyzed in different municipalities of the state of Zacatecas:
San Juan de los Hornillos, municipality of Fresnillo. This community is located between coordinates 23° 36' - 22° 49' N and 102° 29' - 103° 31' W, in the center of the state of Zacatecas. The predominant climates are temperate subhumid with rains in summer (Cw0) and semi-dry with rains in summer (BS); the average annual precipitation is 457 mm, the average annual temperature is 15 °C, the elevation varies from 2 250 to 2 700 m (Medina & Ruiz, 2004) and the predominant slope is 45°.
Sierra de Órganos, municipality of Sombrerete. The study area is located in Sierra de Órganos National Park in the northwest of the state of Zacatecas. The extreme coordinates are: 23° 44’ 58’’ - 23° 48’ 06.39’’ N and 103° 46’ 37’’ - 103° 48’ 57.93” W. The predominant climate is dry (BS1 kw) and in the high parts the climate is Cw0 (Medina & Ruiz, 2004). The average annual temperature is 15 °C, the average annual precipitation is 522 mm (Enríquez, Koch, & González, 2003) and the elevation ranges between 2 120 and 2 650 m.
Concepción del Oro, municipality of Concepción del Oro. The community is located between coordinates 24° 37’ N and 101° 25’ W, in northeastern Zacatecas. The predominant climate is dry or steppe, semi-warm (BS or BW). The average annual precipitation is 420 mm, the average annual precipitation is 17 °C (Medina & Ruiz, 2004) and the elevation ranges between 2 600 and 2 900 m.
Cerro de Piñones, municipality of Juchipila. This site belongs to the Sierras y Valles Zacatecanos physiographic subprovince of the Sierra Madre Occidental province. The extreme coordinates are 21° 20’ - 21° 23’ N and 103° 12’ - 103° 15’ W. The climate is Cw; the average annual temperature is 16 °C and the average annual precipitation is 684 mm (Medina & Ruiz, 2004). The pinyon pine community develops at an elevation of 1 900 to 2 500 m.
Sampling
Floristic composition
In each of the communities, the species with arboreal, shrub and herbaceous habit were collected by the sweep method, including annuals and perennials. Samples were collected during the dry and wet seasons of 2015 and 2016. Identification was made using taxonomic keys (Gentry, 1982; Rzedowski & Rzedowski, 2005) in the Ecology Laboratory of the Forest Sciences Division of Chapingo Autonomous University. The nomenclature of the species was cited based on the terminology established by Tropicos.org (Missouri Botanical Garden, 2017).
Structural characterization of the vegetation
In each community, six lines of 100 m each were established perpendicular to the slope and located in all physiognomic variants; a sampling point was established every 20 m, completing five per line. Vegetation was characterized using the point-centered quarter method (Mueller-Dumbois & Ellenberg, 1974). Sampling sites showed high environmental heterogeneity with difficult-to-access terrain, abrupt topography and steep and pronounced slopes, so the use of sampling plots to estimate density and other structural attributes is not feasible (Kissaa & Sheilb, 2012). The point-centered quarter method is a technique that does not involve the use of a specific area (sampling plots) and considers only the distance between trees, in order to simplify field work without affecting reliability (Zhu & Zhang, 2009). At each point the distance to the nearest species was taken, corresponding to each of the four quarters, considering only species with shrub or arboreal habit with height greater than 1 m; in addition, the diameter at breast height (DBH) and height were recorded. From these data, the relative dominance (RD), density (RDE) and frequency (RF) values of the species were estimated (Mueller-Dumbois & Ellenberg, 1974). The relative importance value (RIV) of each species was calculated with the formula RIV = (RD + RDE + RF) / 3 (Matteucci & Colma, 1982).
The vertical and horizontal structure of the vegetation in each community was represented by means of semi-realistic profile diagrams (Richards, Walsh, Baillie, & Greig-Smith, 1996), prepared based on the RD, RDE, RF, average height of the species and field observations on the physiognomy of the vegetation at the sites. A profile was made for each community based on the most widespread physiognomic variant.
Edaphic composition
In each community, 10 soil samples were collected in the first 10 cm of depth. These were mixed in a composite sample of approximately 1 kg per community. The samples were analyzed and the following parameters were obtained: pH, electrical conductivity (EC), organic matter (OM), nitrogen (N), assimilable phosphorus (P), potassium (K), calcium (Ca) and magnesium (Mg).
Multivariate statistical analysis
Cluster analysis was used to classify plant communities into categories or groups, based on the floristic composition of each community. The clustering was hierarchical, polythetic and agglomerative, using the average among groups as a method of union, and the Sørensen index as a measure of similarity (Rocha, Chavez, Ramírez, & Cházaro, 2012). To define the groups formed in the cluster analysis, a standard cut level was chosen in which 50 % of the remaining information was considered (McCune & Grace, 2002). The influence of the edaphic factors (pH, EC, OM, N, P, K, Ca and Mg) in the floristic composition of the communities was determined with a canonical correspondence analysis. Both analyses were carried out with the PC-ORD version 6 calculation program (McCune & Mefford, 2011).
Results and discussion
Floristic and structural characterization of vegetation in pinyon pine forests
A total of 243 species of vascular plants were recorded in the four pinyon pine communities in the state of Zacatecas. The species belong to 58 families and 167 genera (Appendix 1). The best represented families in number of species were Asteraceae (49), Poaceae (24), Fabaceae (19), Asparagaceae (15) and Cactaceae (13). The general characteristics of each community are described below:
San Juan de los Hornillos, municipality of Fresnillo. In this community, 60 species of vascular plants were recorded, corresponding to 47 genera and 22 families. The community had a density of 1 187 tree and shrub individuals per hectare. Pinus cembroides Zucc. was the most relevant species since it had a higher density and was more frequent (Table 1). This species is part of the tree stratum, along with Yucca filifera Salm-Dyck and Quercus laeta Liebm. as companion species; the average height of the canopy was 3 m, with few emergent individuals of no more than 8 m (Figure 1). In the shrub stratum, the most relevant species were Dasylirion cedrosanum Trel. and Dodonaea viscosa L. Jaqc. The perimeter region of the pinyon pine forest of San Juan de los Hornillos is used as a tourist recreation and livestock foraging area, so the anthropogenic impact is evident, since there is soil loss, pollution and removal of wild plants, mainly. In areas farther from the edge, trees reach bigger sizes and there are more pinyon pines, due to the fact that human settlements are practically abandoned, owing to insecurity and migration, thus reducing the extraction of resources, including the pine nut.
Sierra de Órganos, municipality of Sombrerete. The arboreal vegetation consists of Acacia schaffneri (S. Watson) F. J. Herm., Buddleja cordata Kunth, Juniperus deppeana Steud., Quercus grisea Liebm., Y. filifera and P. cembroides, the last being the most important for the community (Table 1). These species, together, form an open canopy 6 m high, while the shrub stratum, consisting of species such as Ziziphus obtusifolia (Hook. ex Torr. & Gray) Gray, Arctostaphylos pungens Kunth, Dasylirion wheeleri S. Wats. ex Rothr. and Condalia fasciculata I. M. Johnst, does not exceed 2 m (Figure 1). This site, being a protected natural area, is in a relatively better state of conservation. It has the greatest floristic richness with 82 species of 70 genera and 30 families, and it has the lowest arboreal and shrub density with 197 individuals·ha-1; in addition, the stems are larger, indicating the forest’s advanced age (van der Maarel & Franklin, 2013).
Concepción del Oro, municipality of Concepción del Oro. The flora includes 70 species, 54 genera and 22 families, and the density of species with arboreal and shrub habitat is 535 individuals·ha-1. In the arboreal stratum, the plant community presented an association of P. cembroides and P. cembroides var. bicolor Little, the latter with a higher RIV due to its density and basal area. On average, the arboreal stratum composed of Yucca carnerosana (Trel.) McKelvey, Nolina parviflora Kunth and Juniperus pinchotii Sudw is low (5 m, Figure 1); the emergent species is P. cembroides, reaching heights of 10 m. On the other hand, the shrub stratum is poor, D. cedrosanum being the most frequent species (Table 1). In this area another species of pinyon pine is reported: Pinus pinceana Gordon & Glend. (Villarreal, Mares, Cornejo, & Capo, 2009); however, it was not recorded, so it is necessary to expand the sampling area.
Cerro de Piñones, municipality of Juchipila. In this community, 78 species were identified, divided into 69 genera and 38 families, with a density of 1 170 individuals· ha-1. On the plateaus of the Juchipila mountain ranges, forests are dominated by Pinus maximartinezii Rzed. (Table 1), a species in danger of extinction and of restricted distribution. This pinyon pine has populations of 621 individuals·ha-1 and is part of the low arboreal stratum, 4 m on average, in association with Quercus eduardii Trel., Quercus resinosa Liebm., Prosopis laevigata (Humb. & Bonpl. ex Willd.) M. C. Johnst., J. pinchotii and Parkinsonia praecox (Ruiz & Pav.) Hawkins. This area has the highest specific richness in the stratum. On the other hand, the most representative species of the shrub stratum are A. pungens, D. viscosa and Jatropha dioica Sessé (Figure 1). The community is under constant disturbance due to cattle ranching and the gathering of pine nuts, despite which it develops in difficult-to-access areas and under the protection of the inhabitants. In the state of Durango, P. maximartinezii is also distributed (González, González, Ruacho, & Molina, 2011); however, populations that have a discontinuous distribution may have marked genetic differences, so it is important and necessary to establish areas for their conservation and increase the survival of juvenile trees (González-Elizondo et al., 2011; Ledig et al., 2001).
Table 1 Structural values of shrub and arboreal vegetation in four pinyon pine communities in the state of Zacatecas.
| Species | Height (m) | Density (individuals·ha-1) | RD | RF | RDE | RIV |
|---|---|---|---|---|---|---|
| San Juan de los Hornillos, Fresnillo | ||||||
| Pinus cembroides | 4.25 ± 3.09 | 759.68 | 52.44 | 46.00 | 64.00 | 54.15 |
| Dasylirion cedrosanum | 2.00 ± 0.57 | 71.22 | 25.15 | 12.00 | 6.00 | 14.38 |
| Dodonaea viscosa | 1.36 ± 0.36 | 201.79 | 1.33 | 20.00 | 17.00 | 12.78 |
| Yucca filifera | 2.00 ± 0.53 | 71.22 | 7.42 | 12.00 | 6.00 | 8.47 |
| Arctostaphylos pungens | 5.00 ± 0.00 | 11.87 | 11.13 | 2.00 | 1.00 | 4.71 |
| Quercus laeta | 3.34 ± 1.13 | 59.35 | 2.15 | 6.00 | 5.00 | 4.38 |
| Jatropha dioica | 1.10 ± 0.00 | 11.87 | 0.37 | 2.00 | 1.00 | 1.12 |
| Sierra de Órganos, Sombrerete | ||||||
| Pinus cembroides | 6.79 ± 2.68 | 119.91 | 57.51 | 45.65 | 60.87 | 54.68 |
| Juniperus depeana | 4.28 ± 1.20 | 19.27 | 5.02 | 13.04 | 9.78 | 9.28 |
| Quercus grisea | 4.06 ± 0.67 | 17.13 | 7.27 | 8.70 | 8.70 | 8.22 |
| Acacia schaffneri | 3.67± 0.57 | 6.42 | 13.83 | 2.17 | 3.26 | 6.42 |
| Ziziphus obtusifolia | 2.15 ± 0.57 | 8.57 | 1.81 | 8.70 | 4.35 | 4.95 |
| Yucca filifera | 1.92 ± 1.02 | 6.42 | 2.62 | 6.52 | 3.26 | 4.14 |
| Dasylirion wheeleri | 0.75 ± 0.35 | 4.28 | 3.20 | 4.35 | 2.17 | 3.24 |
| Arctostaphylos pungens | 1.90 ± 0.00 | 2.14 | 0.51 | 2.17 | 1.09 | 1.26 |
| Condalia fasciculata | 1.19 ± 0.00 | 2.14 | 0.04 | 2.17 | 1.09 | 1.10 |
| Concepción del Oro, Concepción del Oro | ||||||
| Pinus cembroides var. bicolor | 2.60 ± 1.38 | 289.27 | 11.58 | 39.08 | 54.07 | 34.91 |
| Pinus cembroides | 11.37 ± 3.17 | 71.54 | 34.30 | 9.20 | 13.38 | 18.96 |
| Yucca carnerosana | 4.12 ± 1.95 | 65.32 | 20.39 | 18.39 | 12.22 | 17.00 |
| Dasylirion cedrosanum | 1.49 ± 0.50 | 46.66 | 17.36 | 13.79 | 8.72 | 13.29 |
| Nolina parviflora | 1.76 ± 0.61 | 18.66 | 14.90 | 4.60 | 3.48 | 7.66 |
| Juniperus pinchotii | 3.40 ± 1.99 | 18.66 | 0.91 | 6.90 | 3.47 | 3.76 |
| Ceanothus greggii | 1.55 ± 0.52 | 18.66 | 0.30 | 5.75 | 3.49 | 3.18 |
| Purshia plicata | 0.75 ± 0.07 | 6.22 | 0.26 | 2.30 | 1.16 | 1.24 |
| Cerro de Piñones, Juchipila | ||||||
| Pinus maximartinezii | 4.23 ± 2.51 | 621.56 | 60.83 | 39.02 | 53.13 | 50.99 |
| Dodonaea viscosa | 2.28 ± 0.84 | 237.66 | 3.58 | 19.51 | 20.31 | 14.47 |
| Arctostaphylos pungens | 2.84 ± 0.74 | 109.69 | 10.92 | 14.63 | 9.38 | 11.64 |
| Quercus eduardii | 4.25 ± 2.47 | 36.56 | 14.55 | 4.88 | 3.12 | 7.52 |
| Parkinsonia praecox | 3.50 ± 2.12 | 36.56 | 5.53 | 4.88 | 3.12 | 4.51 |
| Prosopis laevigata | 2.23 ± 0.68 | 54.84 | 1.43 | 7.32 | 4.69 | 4.48 |
| Juniperus pinchotii | 4.20 ± 1.13 | 36.56 | 1.78 | 4.88 | 3.12 | 3.26 |
| Jatropha dioica | 1.70 ± 0.00 | 18.28 | 0.52 | 2.44 | 1.56 | 1.51 |
| Quercus resinosa | 4.17 ± 0.00 | 18.28 | 0.86 | 2.44 | 1.56 | 1.62 |
± Standard deviation of the mean. RD: relative dominance, RF: relative frequency, RDE: relative density and RIV: relative importance value.

Figure 1 Semi-realistic profile of four pinyon pine sites in the state of Zacatecas: a) San Juan de Hornillos, Fresnillo; b) Sierra de Órganos, Sombrerete; c) Concepción del Oro (dominated by Pinus cembroides var. bicolor); d) Concepción del Oro (dominated by Pinus cembroides); e) Cerro de Piñones, Juchipila. Species: 1. Pinus cembroides, 2. Yucca filifera, 3. Arctostaphylos pungens, 4. Dodonaea viscosa, 5. Agave spp., 6. Nolina parviflora, 7. Jatropha dioica, 8. Opuntia sp., 9. Quercus laeta, 10. Dasylirion cedrosanum, 11. Juniperus deppena, 12. Ziziphus obtusifolia, 13. Vachellia schaffneri, 14. Quercus eduardii, 15. Dasylirion wheeleri, 16. Condalia fasciculata, 17. Quercus grisea, 18. Juniperus pinchotii, 19. P. cembroides var. bicolor, 20. Celtis pallida, 21. Yucca carnerosana, 22. Purshia plicata, 23. Quercus resinosa, 24. Parkinsonia praecox, 25. Prosopis laevigata.
Classification and ordination of pinyon pine forests
Structurally, in the pinyon pine forests of the state of Zacatecas, three physiognomic variants can be distinguished: Pinus cembroides forests, the P. cembroides - P. cembroides var. bicolor association and the forest dominated by P. maximartinezii; however, floristically, the cluster analysis showed two well-defined groups at a cut-off level with 50 % remaining information (Figure 2).

Figure 2 Hierarchical dendrogram showing the floristic similarities among four pinyon pine communities in the state of Zacatecas.
According to Figure 2, the forests of P. cembroides and P. cembroides-P. cembroides var. bicolor have greater floristic affinity with each other than with the forest dominated by P. maximartinezii. This floristic difference may be due to the geographic distance between the sites, since the closest communities could present similar environmental conditions in comparison with the more distant ones; that is, the floristic similarity decreases with the increase in distance (Tuomisto, Ruokolainen, & Yli-Halla, 2003), as has been reported in forests dominated by P. pinceana (Villarreal et al., 2009).
Soil composition is a relevant factor for determining structural differences in plant communities (Chavoya et al., 2016; Granados et al., 2015; Sánchez-González & López-Mata, 2003; Sardinero, 2000). The canonical correspondence analysis determined that the predominant edaphic factors in the floristic composition of the pinyon pine forests are pH, EC, K concentration and soil texture, especially the percentage of clay (Table 2; Figure 3).
Table 2 Correlation between the edaphic variables of the pinyon pine communities in the state of Zacatecas and the ordination axes of the canonical correspondence analysis.
| Variable | Axis 1 | Axis 2 | Axis 3 |
|---|---|---|---|
| pH | -0.916 | -0.065 | -0.088 |
| Electrical conductivity | -0.890 | -0.088 | -0.141 |
| Organic matter | -0.361 | 0.200 | 0.174 |
| N | 0.568 | 0.482 | -0.181 |
| P | -0.321 | 0.355 | -0.542 |
| K | 0.840 | 0.187 | 0.062 |
| Mg | 0.700 | -0.187 | -0.046 |
| Fe | 0.316 | -0.316 | 0.618 |
| Sands | -0.236 | -0.073 | 0.318 |
| Silts | -0.114 | 0.544 | -0.537 |
| Clays | 0.746 | -0.485 | 0.035 |
| Total variance | |||
| Eigenvalue | 0.880 | 0.818 | 0.374 |
| Species - environmental factors correlation | 1 | 1 | 0.988 |
| Explained variation (%) | 27.9 | 25.9 | 11.8 |
| Cumulative variance (%) | 27.9 | 53.8 | 65.6 |
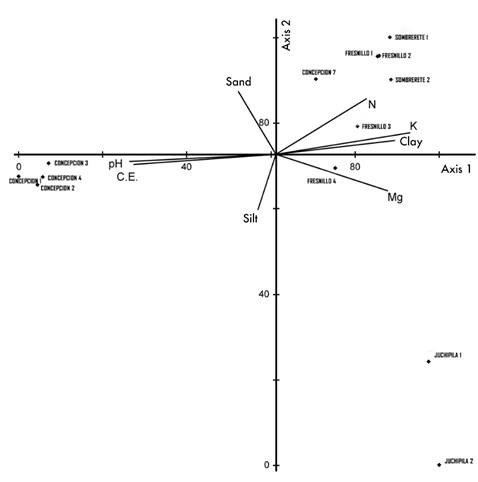
Figure 3 Ordination diagram based on the canonical correspondence analysis of the sampling sites with respect to the soil factors of the pinyon pine communities analyzed in the state of Zacatecas.
Table 3 shows the physicochemical properties of the analyzed soils. The forests dominated by P. cembroides (Fresnillo and Sombrerete) develop on soils with slightly acid pH (5), low EC, low concentration of Ca, and rich in K and N. Acid pH values are common in pine forests due to the decomposition process of the organic waste (Granados, López, & Hernández, 2007). On the other hand, communities with codominance of P. cembroides var. bicolor and P. cembroides inhabit sites with neutral pH (7) and higher EC due to the high Ca concentration (Osman, 2013). The plant species found in the Concepción del Oro community have relatively greater resistance to salinity and nutrient-poor soils (Gandullo, 2004). In the case of P. maximartinezii, the forest is located in the zone of greatest precipitation and lowest elevation with respect to the other communities in the state of Zacatecas. The soils are slightly acid (5.9) with loam texture, considered the most propitious for plant growth, since it favors infiltration and good drainage; it also has high fertility and adequate water retention due to its proportion of sand, silt and clay (Osman, 2013; Rodríguez-Fuentes & Rodríguez-Absi, 2002).
Table 3 Physicochemical soil properties of the pinyon pine communities in the state of Zacatecas.
| Site | pH | EC | OM | N | P | K | Ca | Mg | Fe | Sand | Silt | Clay | Texture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (dS·m-1) | (%) | ----------------------(mg·kg-1) --------------------- | ------------------(%)------------------- | ||||||||||
| Fresnillo | 5.305 | 0.06 | 9.56 | 10.22 | 4.63 | 448.5 | 1 969.0 | 394.7 | 59.69 | 75.7 | 14.8 | 9.5 | Sandy loam |
| Sombrerete | 5.810 | 0.04 | 2.82 | 18.75 | 13.76 | 391.0 | 1 492.5 | 239.0 | 46.95 | 71.1 | 17.6 | 11.3 | Sandy loam |
| Concepción del Oro | 7.114 | 0.13 | 6.54 | 9.68 | 17.40 | 126.0 | 4 589.0 | 173.2 | 25.36 | 65.6 | 26.3 | 7.9 | Sandy loam |
| Juchipila | 5.595 | 0.05 | 3.42 | 10.15 | 2.66 | 340.0 | 1 418.5 | 551.0 | 47.49 | 47.2 | 42.3 | 10.5 | Loam |
EC: electrical conductivity; OM: organic matter
Environmental factors such as temperature and slope of the land were rejected because the four communities had a certain climatic and orographic affinity, and in other regions of the country, with the same type of vegetation, these variables were not considered relevant to explain the assembly of species (Chavoya et al., 2016; Romero et al., 2014; Romero-Manzanares, Flores-Flores, Luna-Cavazos, & García-Moya, 2016).
Conclusions
The floristic richness of the pinyon pine forests studied in Zacatecas was 244 species, presenting between 60 and 80 species per community; however, the floristic similarity was low among the four communities, with different dominance, frequency and density relationships. This is the result of the multifactorial interaction of environmental and edaphic factors with the species, so each of the communities must be managed differently, meeting the particular requirements of the species that develop there.











 texto en
texto en 


