Introduction
The arbuscular mycorrhizal association is a symbiosis between fungi and the root system of plants. It is a biotrophic, harmonious and non-pathogenic interaction that contributes to maintain ecosystems in a sustainable manner (Bonfante & Genre, 2012; Scheublin & van der Heijden, 2006). The described symbiosis is mutualistic, where both organisms benefit from a reciprocal exchange of minerals and organic resources (Cuenca, Cáceres, Oirdobro, Hasmy, & Urdaneta, 2007) to improve the nutritional status of the parties involved.
Mycorrhizal fungi facilitate the absorption of nutrients that are hardly available to plants (Ortas, 2009; van der Heijden & Scheublin, 2007); in addition, they favor osmotic adjustment during periods of stress due to a lack of moisture in the soil (Augé et al., 2004). On the other hand, fungi require the host plant to complete the life cycle. This is how the plant controls the colonization and formation of arbuscules according to their needs, regulates their defense mechanisms and initiates a communication process preparing the cells of the root cortex to receive the symbiote (Schmitz & Harrison, 2014). As colonization progresses through the root, the fungal structures develop into two types of association called Arum and Paris. In the first, the hypha grows intercellularly in the cortex, longitudinally to this tissue and penetrates with short branches in the cells forming arbuscules in its interior. In the Paris type, the intracellular coiled hyphal; it does not always originate arbuscules capable of interacting with the cytoskeleton. In this way, the hypha remains separated from the cytoplasm of the host cell by a matrix at the interface formed in the apoplastic compartment, similar to the type Arum (Kubota, McGonigle, & Hyakumachi, 2005).
Smith and Smith (1997) determined the type of association between arbuscular mycorrhizal fungi (AMF) and plants, showing that the Paris type prevailed in 41 botanical families, while in another 30 families the type Arum was present; in addition, they found intermediate types in 21 families, among them, the Fabaceaes. It has been reported that individuals of this botanical family are able to establish symbiotic relationships with various organisms, both with the AMF and with the bacteria responsible for fixing nitrogen (Zhu, Riely, Burns, & Ané, 2006). The triple symbiotic relationship plant-fungi-bacteria guarantees greater efficiency to the legumes, increasing their biological importance (Scotti & Corrêa, 2004).
The tree species Pithecellobium dulce (Roxb.) Benth. and Platymiscium diadelphum S. F. Blake are native plants capable of withstanding the adverse conditions that characterize the semiarid zone of Lara, Venezuela. The fresh seeds of P. dulce and P. diadelphum reach emergency values of 91 and 78 %, respectively, which increases their potential to include them in urban landscape and with ecological and economic benefits, such as the production of excellent quality wood in the case of oak (Parra, Sanabria, & Maciel, 2011). A strategy that guarantees the survival of tree species, given the stress conditions of transplanting and establishment in urban wooded areas, is the inoculation with AMF at the initial stages in nursery.
The objectives of the present study were to describe the colonization of the AMF Rhizophagus manihotis (R. H. Howeler, Sieverd. & N. C. Schenck) C. Walker & A. Schüßler, Funneliformis mosseae (T. H. Nicolson & Gerd.) C. Walker & A. Schüßler and Scutellospora heterogama (T. H. Nicolson & Gerd.) C. Walker & F. E. Sanders, inoculated in plants of yacure (P. dulce) and oak (P. diadelphum), trees adapted to semiarid conditions; and determine the morphological type of the symbiotic association.
Materials and methods
The experiments were carried out in the facilities of the Posgrado del Decanato de Agronomía of the Universidad Centroccidental “Lisandro Alvarado” (10º 01’ 30’’ N, 69º 16’ 30’’ W and 500 m of altitude) located in Cabudare, in the municipality of Palavecino in the state of Lara, Venezuela. The annual precipitation of the place is 662 mm with average annual temperature of 24.9 °C.
The mature fruits of P. dulce and P. diadelphum were collected from 12 and 25 years old adult trees, respectively, which are located in the facilities of the institution. The fruits were processed to select seeds based on size, degree of formation, coloration, absence of malformations and apparent symptoms of diseases. In containers designed to produce forest trees, substrate with mixture of coconut sawdust, rice husk and sandy loam (1: 1: 1) pasteurized was added. The substrate was placed until completing 70 % of the available volume of the cell; each one with 30 g of granulated inoculum of AMF, conforming the following treatments: R. manihotis (T1), F. mosseae (T2) and S. heterogama (T3). A seed was sown in each container. The pure strains of AMF were provided by the Unidad Micoven of the Instituto Venezolano de Investigaciones Científicas (IVIC).
The design of experiments was completely random; each treatment consisted of 40 experimental units. At 105 days after sowing (das), the secondary roots of 20 plants, selected at random, were subjected to the clarification process with the methodology of Phillips and Hayman (1970). The roots were divided into two groups and stained with acid fuchsin and trypan blue.
The percentage of colonization in the roots was determined with the methodology proposed by McGonigle, Hiller, Evans, Fairchild, and Swan (1990); the thinnest ones (<1 mm) were chosen and separated into 1 cm long sections. The roots were placed on the slide in vertical position. Colonization was described through observations with an optical microscope (Olympus CX21, China) of 400x and 1000x magnification. The mycorrhizal structures (hyphae, arbuscules and vesicles) were quantified by the following formulas:
Colonization (%): [colonized segments (cm) / (uncolonized segment (cm) + colonized segment (cm)] * 100
Colonization by hyphae (%): (number of segments with hyphae / number of total segments) * 100
Colonization by arbuscules (%): (number of segments with arbuscules / number of total segments) * 100
Colonization by vesicles (%): (number of segments with vesicles / number of total segments) * 100
The stained roots were cross-sectioned by freehand. Fungal structures were described in each segment and section. The information was necessary to define the morphological type of the association.
Statistical analysis
The colonization percentage data was analyzed by analysis of variance after checking the statistical assumptions of the completely random design, using the program Statistix version 8.0. (Analytical Software, 2003). Subsequently a comparison of means was made with the Dunnet test (P ≤ 0.05).
Results and discussion
Pithecellobium dulce
Colonization of arbuscular mycorrhizal fungi at 105 days after sowing
Table 1 shows the results of colonization and development of AMF structures in P. dulce. At 105 das, R. manihotis colonized 80.36 % of the roots and developed 79.40 % of hyphae and 47.60 % of arbuscules; F. mosseae colonized 61.38 % and generated 61.05 % of hyphae and 31.57 % of arbuscules; while S. heterogama had approximately the same percentage of colonization, hyphae and arbuscules (59 %). The analysis of variance detected significant differences (P < 0.05) between at least a pair of means of the treatments inoculated when estimating the variables mentioned above, being R. manihotis (T1) the one with highest percentage of colonization and hyphae. The production of arbuscules and vesicles was statistically similar (P > 0.05) in R. manihotis and S. heterogama. Rhizophagus manihotis and F. mosseae had 18.91 and 15.95 % intra-radicular vesicles, respectively. It should be noted that S. heterogama does not produce them (González & Cuenca, 2008). The vesicles are indicative of the end of the development of mycorrhizal fungi when colonizing the roots (Bonfante & Genre, 2012), so that it is inferred that R. manihotis and F. mosseae kept growing actively.
Table 1 Colonization and structures of arbuscular mycorrhizal fungi inoculated in Pithecellobium dulce. The fungal structures were quantified in root segments at 105 days after sowing.
| Treatments | Colonization (%) | Hyphas (%) | Arbuscules (%) | Vesicles (%) |
|---|---|---|---|---|
| Rhizopagus manihotis | 80.36 ± 2.62 a | 79.40 ± 2.85 a | 47.60 ± 3.67 a | 18.91 ± 2.25 a |
| Funneliformis mosseae | 61.38 ± 2.91 b | 61.05 ± 3.01 b | 31.57 ± 1.88 b | 15.95 ± 2.67 a |
| Scutellospora heterogama | 59.98 ± 3.81 b | 59.38 ± 3.90 b | 59.38 ± 2.33 a | 0 a |
| Coefficient of variation (%) | 12.26 | 12.74 | 17.08 | 19.03 |
Means with different letter in each column differ significantly according to the Dunnet test (P ≤ 0.05).
The highest percentage of colonization corresponded to the treatment where R. manihotis (80.36 %) was inoculated. In the case of F. mosseae and S. heterogama, although they were inferior to those of T1 (61.38 and 59.58 %, respectively), they exceeded the value indicated by Habte, Fox, Aziz, and El-Swaify (1988) for L. leucochepala ‘K8’, inoculated with Glomus agregatum N. C. Schenck & G. S. Sm. (30 % colonization). This suggests that the spores used in the tests had adequate conditions for germination and, as indicated by these authors, the fungi behaved like good colonizers. The results demonstrated the affinity between AMF and P. dulce, a species belonging to the fabaceae, which coincided with the results obtained by Patreze and Cordeiro (2005), who indicated 24.72, 42.25 and 15.35 % of colonization of native mycorrhizal fungi in Enterolobium. contortisiliquum (Vell.) Morong, Inga laurina (Sw.) Willd. and Platypodium elegans Vogel, respectively. The values superior to 50 % of colonization, in mycorrhizal relations, favor the ecological succession and level of survival, as indicate by Kumar, Raghuwanshi, and Upadhyay (2003) in Acacia auriculiformis A. Cunn. ex Benth., A. catechu (Willd.) Wight & Arn., A. procera (Roxb.) Willd., A. nilotica (L.) Delile, Albizia lebbeck (L.) Benth., Prosopis juliflora (SW) DC., Pongamia pinnata (L.) Pierre, Dalbergia sissoo Roxb. ex DC. and Tamarindus indica species of the Fabaceae family.
With respect to the percentage of arbuscules, the fluctuation between the treatments with R. manihotis, F. mosseae and S. heterogama in P. dulce is justified, since these structures are short-lived, with seasonal occurrence and formation sensitive to variation of environmental factors and physiological metabolism of the host plant (Alexander, Meier, Toth, & Weber, 1988).
On the other hand, the low percentage of vesicles determined in this study (18.95 and 15.95 % for R. manihotis and F. mosseae, respectively) is explained by the fact that the formation of these storage structures depends on the type of mycorrhizal fungi, of its development within the root and environmental factors (Hawley & Dames, 2004; Kumar et al., 2003).
Anatomy of arbuscular mycorrhizal fungi colonization
Figure 1 illustrates the colonization of the arbuscular mycorrhizal fungi R. manihotis (A and E), S. heterogama (B and G) and F. mosseae (C, D and F). The colonization in P. dulce was evidenced in the terminal roots, maintaining the integrity of the cortical cells. The hyphae formed thickened hifopodia, which were the means of penetration through or between the rhizodermal cells (Figure 1B). These observations coincided with those described by Garriock, Petersonm, and Ackerley (1989), who considered that these structures are similar to those of pathogenic fungi. The inter and intracellular hyphae developed from the penetration points (Figures 1B and 1D) in circumferential and longitudinal directions, crossing the cortical parenchyma, without reaching the vascular cylinder (Figures 1F and 1G).
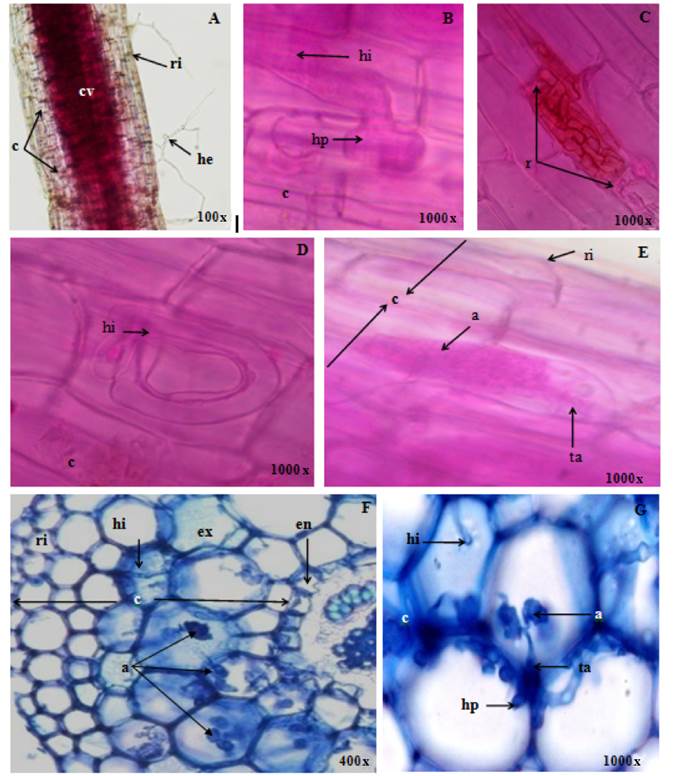
Figure 1 Colonization of the arbuscular mycorrhizal fungi Rhizopagus manihotis (A and E), Scutellospora heterogama (B and G) and Funneliformis mosseae (C, D and F) in pruning (A, B, C, D and E) and cross sections (F and G) of Pithecellobium dulce roots; where rhizodermis (ri), cortex (c), vascular cylinder (cv), extrarradical hyphae (he), hifopodium (ap), intraradical hypha (hi), intracellular (r), exodermis (ex), endodermis (en), arbúsculo (a) and trunk or base of the arbuscle (ta) can be observed.
According to the development of the intraradical mycelium, the morphological behavior observed in P. dulce was intermediate between Arum (Figures 1A, 1B and 1F) and Paris (Figures 1C and 1D). During the first phase of colonization, the second morphological type was predominant in the circumferential direction, since the fungal symbiont propagated in the cortical parenchyma by means of intracellular hyphae of considerable thickness (Figure 1F); subsequently, arbuscules were formed as terminal structures within the cells, in addition to intercellular spirals (Figure 1C). The arbuscular interfaces in Arum and Paris were similar. In the arbuscules, the trunk or base could be distinguished, while the ends were divided dichotomically (Figure 1E). These results coincided with those reported by Shi, Chen, Feng, Liu, and Christie (2006) in species of Meliaceae, and with those of Armstrong and Peterson (2002) in Panax quinquefolius L. Although the function of hyphae rolled into the type Paris is still uncertain, Cavagnaro, Smith, Ayling, and Smith (2003) stated that they provide an interfacial area as wide as arbuscules, which could be related to the transfer of nutrients in plant species tolerant to environmental stress and slow growth. On the other hand, Hawley and Dames (2004) considered that the host could determine the dominant morphological type, indicating that the different structures are no more than adaptations for fungi survirval.
Platymiscium diadelphum
Colonization of arbuscular mycorrhizal fungi at 105 days after sowing
Table 2 presents the results of the colonization of R. manihotis, F. mosseae and S. heterogama in P. diadelphum at 105 das. The colonization of the three AMF was greater than 90 %, being statistically similar (P > 0.05). The percentage of hyphae, arbuscules and vesicles was also similar in the three treatments.
The interaction and bidirectional exchange between the plant and the mycorrhizal fungi were evidenced with a satisfactory percentage of arbuscules in each treatment. The lowest value was 69.7 % in F. mosseae, while the highest corresponded to R. manihotis (85.7 %); all three treatments were statistically similar (P > 0.05). The importance of the presence of arbuscules lies in the fact that these structures participate directly in the transport of compounds necessary for the development of the plant and fungi (Ortas, 2009).
The percentages of intraradical vesicles were 85.7 and 12.2 for R. manihotis and F. mosseae, respectively. These values allowed to infer that the interaction between P. diadelphum and the inocula used if it was carried out, since the colonization was greater than 90 %. Despite the difference margins of the values, the analysis of variance (P < 0.05) did not detect significant differences between the variables evaluated. The low values are due to the fact that the vesicles are formed with the purpose of storing reserve substances, so they are less perceived until fungi are fully established.
Table 2 Colonization and structures of arbuscular mycorrhizal fungi inoculated in Platymiscium diadelphum. The fungal structures were quantified in root segments at 105 days after sowing.
| Treatments | Colonization (%) | Hyphas (%) | Arbuscules (%) | Vesicles (%) |
|---|---|---|---|---|
| Rhizopagus manihotis | 98.50 ± 2.75 | 93.50 ± 2.99 | 85.70 ± 3.24 | 85.70 ± 2.47 |
| Funneliformis mosseae | 92.40 ± 2.95 | 91.10 ± 3.07 | 69.70 ± 3.88 | 12.20 ± 3.35 |
| Scutellospora heterogama | 91.30 ± 2.37 | 88.10 ± 3.65 | 72.20 ± 3.83 | 0.0 |
| Coefficient of variation (%) | 17.22 | 6.97 | 12.42 | 18.03 |
According to the Dunnet test, there was no significant difference (P> 0.05) in the effect of arbuscular mycorrhizal fungi (treatments).
The results showed that the level of association of P. diadelphum with R. manihotis, F. mosseae and S. heterogama is high, regarding the classification categories proposed by Kumar et al. (2003).
The mycorrhizal relationship of P. diadelphum agrees with that expressed by Saslis-Lagoudakis, Chase, Robinson, Russell, and Klitgaard (2008), who determined that the success in the establishment of Platymiscium is due to the development of strategies to optimize development and adaptation in dry biomes, in turn Smith and Smith (1997) indicated the existence of a mycorrhizal symbiosis in the legumes of the subfamily Papilionoideae.
For individuals of the Fabaceae family, high percentages of colonization by mycorrhizal fungi have been reported. In this same sense, in a study carried out in six families of angiosperms, Fabaceae ranked third in colonization with values higher than 50 % (Ali, Mubassara, Rahman, Alam, & Khan, 2008). Similarly, Rey, Chamorro, and Ramírez (2005) determined superior colonization of 60 % when they used double inoculation with strains of rhizobia in Leucaena leucocephala (Lam.) de Wit, while Ingleby, Wilson, and Munro (2007) reported that the arboreal fabaceae Senna siamea (Lam.) H. S. Irwin & Barneby, Gliricidia sepium (Jacq.) Kunth ex Walp. and Calliandra calothyrsus Meisn, are highly dependent on mycorrhizae and, therefore, respond satisfactorily to inoculation. On the other hand, Sylvia, Alagely, Kent, and Mecklenburg (1998) determined that plants that grow in containers have a greater percentage of colonization than those that grow in open spaces, as observed in Acacia farnesiana (L.) Willd.
The percentages of arbuscules in P. diadelphum were high, corresponding to the moderate to very high categories according to Kumar et al. (2003), although these structures are considered short-lived (Alexander et al., 1988). Opposite situation occurred with the vesicles; the absence of these was attributed to the fact that fungi are in an active phase of development (Hawley & Dames, 2004).
The inoculation with AMF caused satisfactory results; however, there was no constant prevalence of one mycorrhizal fungus over another, although the colonization rates were high. This result allows us to infer that a host plant can interrelate with different AMF, a response that can be measured through the effects on the survival and diversity of plant species in an ecosystem (Scotti & Corrêa, 2004). It is necessary to consider the extrinsic factors of the plant (Jansa, Smith, & Smith, 2008), since the quantification of fungal structures within the root is variable.
Each fungal species has potential characteristics to establish the symbiosis, such is the case of the species of Gigasporaceae that are slow to colonize, but their hyphae reach greater extension in the soil during the germination of the spore and after this period (Matekwor, Andoh, & Nonaka, 2007). The dynamics of growth and development of the host plant and the mycorrhizal fungus simultaneously regulates bidirectional exchange; therefore, synchronization depends on various factors that define the complexity of the relationship (van der Heijden & Scheublin, 2007).
Anatomy of colonization
Figure 2 shows the colonization of the arbuscular mycorrhizal fungi S. heterogama (A and C), R. manihotis (B and H) and F. mosseae (D, E, F and G). In the longitudinal and transverse sections of roots of P. diadelphum a large volume of thin, transparent and cenocitic hyphae was observed, surrounding the terminal roots (Figure 2A).
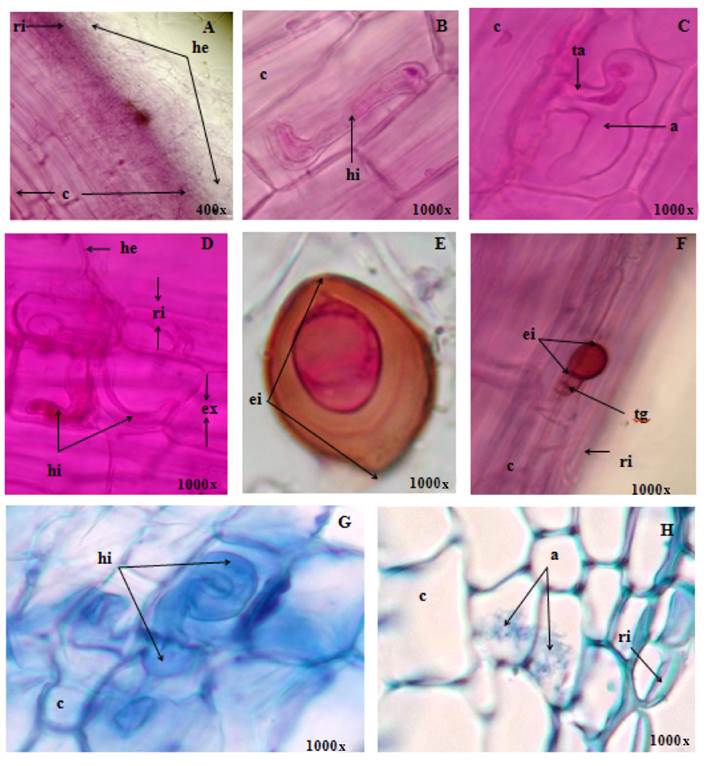
Figure 2 Colonization of the arbuscular mycorrhizal fungi Scutellospora heterogama (A and C), Rhizopagus manihotis (B and H) and Funneliformis mosseae (D, E, F and G) in rinse (A, B, C, D, E and F) and transverse sections (G and H) from roots of Platymiscium diadelphum; where we can see rhizodermis (ri), exodermis (ex), bark (c), extrarradical hypha (he), intraradical hypha (hi), arbuscule (a), intrarradical spore (ei) and germinative tube (tg) and trunk or base of the arbúsculo (ta).
In the roots the presence of spores and hifopodia in the rhizodermis was evidenced, which initiated the intraradical colonization (Figures 2C, 2E and 2F); subsequently, the hyphae branched extending from the exodermis to the region of the central cylinder (Figure 2H). Sbrana and Giovannetti (2005) and Zhu et al. (2006) stated that germination of the spore and the formation of hifopodia represent the main specific morphogenetic event, whose differentiation is mediated by unknown signals, which probably includes the chemical and mechanical sensitive, specific to the type of hyphae.
During intraradical colonization in P. diadelphum, the hyphae of AMF showed development patterns corresponding to intermediate types between Paris (Figures 2D and 2G) and Arum (Figure 2H). Colonization spread from one cell to another and spirals formed to produce intercellular rollers (Figures 2D and 2G); these characteristics corresponded to the type Paris. The development of the hyphae extended longitudinally intercellularly and at the level of the bark. The hyphae formed, at certain intervals, lateral branches that penetrated the cell wall; they branched dichotomically causing arbuscules (Figure 2C); and, despite the ephemeral life cycle, they followed the pattern established for the Arum morphological type. The arbuscular interface was similar in the Arum and Paris morphological types.
In the opinion of Armstrong and Peterson (2002), the coiled hyphae of Paris interact with the cytoskeleton and are separated from the cytoplasm of the host cells by an interfacial matrix, formed in the apoplastic compartment, showing this similarity with Arum mycorrhiza. It should be noted that the interface of the intercellular hyphae contributes significantly to the release of phosphorus to the host cells.
Apparently, the expression of one or another morphological development of AMF is strongly controlled by the host plant (Matekwor et al., 2007). Urcelay, Tecco, and Chiarini (2005) affirmed that the intermediate types between Arum and Paris present characteristics formed by the same AMF species and that the formation of mycorrhizal structures in the roots is determined by the interaction of the species of plants and fungi involved . The intermediate type of morphological development of AMF observed in P. diadelphum coincided with that reported by Smith and Smith (1997) for Fabaceae. On the other hand, Cavagnaro et al. (2003) considered that this is the most common morphological behavior.
The Paris-type association has been determined more frequently in trees and plants of semi-arid ecosystems (Yamato & Iwasaki, 2002), a characteristic present in P. diadelphum.
Conclusions
The arbuscular mycorrhizal fungi (AMF) Rhizopagus manihotis, Funneliformis mosseae and Scutellospora heterogama established high symbiotic association with Pithecellobium dulce and Platymiscium diadelphum, which is why inoculation of these is recommended in the nursery phase. The morphological association in P. dulce and P. diadelphum inoculated with AMF was of Paris type, mainly, with occasional presence of Arum. In P. dulce, S. heterogama tended to produce more arbuscules; structures that allow bidirectional exchange between the arbuscular mycorrhizal fungi and the host plant. In the case of P. diadelphum, the colonization of the three fungi inoculated and the development of fungal structures were similar.











 texto en
texto en 


