Introduction
The reservoir of nutrients and their movement in the forests is a basic knowledge that allows us to know if a harvesting system is sustainable (Johnson, Sogn, & Kvindesland, 2000). According to the literature, the export of nutrients due to forest harvesting does not threaten sustainability, since some of these are released by the parental soil material (Binkley & Fisher, 2013; Sollins et al., 1980), they return to the soil through leaf litter fall and have high retranslocation rates in the leaf mass; and forest species in general have a low demand of nutrients (Imbert, Blanco, & Castillo, 2004; Machado, Sampaio, Ferraz, Camara, & Pereira, 2016). However, it should not always be assumed that forest harvest does not affect the productive level of the land; rather it should be evaluated, since the lack of information on this subject leaves more uncertainty, especially in the case of Mexico where information is scarce.
In the canopy, nutrients are stored in trunk, branches and foliage. The duration of foliage before the fall and reabsorption of nutrients determine the average time that these reside in the aerial biomass before returning to the soil. In this respect, Rodríguez-Sanfuentes and Álvarez-Muñoz (2010) indicate that the speed of nutrient cycles influences the productivity of the forest. Although it is not always possible to study all the reservoirs and transfers, is important to measure the main soil fluxes to vegetation and vice versa, since it helps to understand the functioning of a forest ecosystem (Imbert et al., 2004).
In old forests, the largest reservoir of nutrients is in soil (Schlesinger & Bernhardt, 2013); however, the availability of some of these depends on the rate of mineralization of leaf litter more than on the magnitude of the reservoir (Grigal, 2000; Landsberg & Gower, 1997). It should be noted that inadequate forest harvesting can deplete nutrient reservoirs (Imbert et al., 2004; Landsberg & Gower, 1997; Maynard et al., 2014), due to the removal of biomass and the vulnerability of soil to erosion and compaction (Gomez, Powers, Singer, & Horwath, 2002; Grigal, 2000; Imbert et al., 2004); consequently, productivity may also be affected (Goya, Pérez, Frangi, & Fernández, 2003; Grigal, 2000).
The objectives of this study were: to evaluate the nutrient reservoirs (N, P, K, Ca and Mg) in trees, litter and mineral soil (at a depth of 80 cm) in two stands of Pinus montezumae Lamb. using the Mexican Method of Irregular Forest Management; estimate the decomposition rate of litter; and quantify the annual amount of nutrients that a mature stand of P. montezumae demands.
Materials and methods
Study area
The study was carried out at San José Nanacamilpa Ejido (19° 29’ 0’’ N and 98° 32’ 0’’ W), municipality of Nanacamilpa, Tlaxcala. According to the weather station of Nanacamilpa (29017), average annual temperature is 8 °C and average annual precipitation is 744 mm (Eric III, 2013). The two stands are composed of pure masses of P. montezumae and the understory is represented by Symphoricarpos microphyllus H. B. K., Baccharis conferta Kunth. and species of the genus Senecio, Salvia, Ceanothus and Muhlenbergia. Slope, exposure and altitude of the stands are: 4 %, Northeast and 2 600 m, respectively, in stand I; and 8 %, Southwest and 2 900 m, respectively, in stand II. The forest has been subject to management using the Mexican Method of Management for Irregular Forests (Hernández-Díaz, Corral-Rivas, Quiñones-Chávez, Bacon-Sobbe, & Vargas-Larreta, 2008). The average annual volume harvested is 5.9 m3·ha-1, which is equivalent to approximately 3 % of the total tree volume.
Sampling site
A total of three circular sites of 0.1 ha were delimited in each stand; one site to the center and two complementary sites located at 50 m. In the three sampling sites, a soil profile of 2 × 1 × 1 m long, wide and deep, respectively, was dug and samples were taken per horizon, to analyze and estimate the mass of nutrients. The diameter at breast height (cm) and total height (m) of all the trees, of the three sites of each stand, were measured using a diametric tape and Haga clinometer, respectively.
Aerial biomass
In the stands studied, 10 trees with a diameter at breast height between 40 and 55 cm were harvested to estimate the aerial biomass. The trunk was sectioned into 2.5 m logs at the base of the tree and 1.25 m at the top, later wood volume was calcultaed with the Smalian formula (Romahn & Ramírez, 2010). The dry biomass per unit of green volume was determined from three wood slices of 5 cm thickness, taken along the trunk. A sub-sample was taken from each wood slice and dried in an oven with forced circulation air (Sheldon Fx14-S, USA) at 85 °C until constant weight.
Branches and foliage of each tree were separated and weighed in green, directly in the field, using a portable hook scale with a capacity of 15 kg and precision of 0.1 kg (Pasola). Separately, three subsamples of approximately 1 kg of foliage and 2 kg of branches were taken and weighed with a balance with a capacity of 2 kg and precision of 0.1 (Ohaus Scout Pro, USA). The subsamples were dried in an oven at 70 °C to estimate the moisture content and then transform the field weight to oven dry weight. The oven dry weight of plant components was obtained with the following formula:
where
PSc |
oven dry weight, in the field |
Phc |
wet weight, in the field |
Fh |
moisture fraction (wet weight/dry weight), in laboratory |
The proportion of total aerial biomass assigned to trunk, branches and foliage was estimated using the total biomass of each tree. Volume was estimated in the three sites of each stand with the diameters at breast height and the total tree heights, using the equation for total tree volume. Tree mass was determined using the volume and wood density.
Forest floor
A total of three leaf litter samples from the forest floor (superficial organic horizon) were obtained in each stand, using a circular frame of 30 cm in diameter (0.071 m2). A sampling point was located at the center of the stand and, approximately at 25 m, two others were located on both sides in a position perpendicular to the slope. Each sample was weighed in the field, then a sub-sample was selected and oven-dried for 72 h at 70 °C in an oven with forced air circulation (Riossa HCF-4848D, Mexico). Once the wet and dry weights were known, the amount of biomass existing in the forest floor was estimated.
Mineral soil
The samples were obtained in each horizon of the soil profiles until reaching the C-horizon depth, which was found at 115 and 95 cm in stand I and II, respectively. Only the first three horizons were sampled in the complementary sites, where the greatest amount of nutrients is concentrated. Preliminary soil determinations, carried out in the area, indicated that the greatest variability is found in the superficial soil. Samples were also taken to obtain the soil bulk density using a sliding hammer (AMS Inc., Idaho, USA) with 98.17 cm3 cylindrical soil cores. The soil samples were oven-dried at 105 °C for 12 h in an oven with forced circulation air (Riossa HCF-4848D); the bulk density was determined with the volume information and soil dry mass. Each of the profiles were analyzed per horizon, according to the Official Mexican Standard NOM-021-RECNAT-2000 (SEMARNAT, 2002) to determine: texture, pH, cation exchange capacity, organic matter, total N, extractable P (Bray method 1) and exchangeable cations (K, Ca and Mg) in ammonium acetate (pH 7). Regarding the depth of the horizons and soil bulk density, the amount of nutrients was estimated at the same depth of 80 cm for each stand. Most reservoirs of nutrients in forests are found at this soil depth (Binkley & Fisher, 2013).
Leaf litter production decomposition
The fall of leaf litter was collected in approximate periods of one month during a year. For this, three litter square traps of 1 m2 were place at ground level on each stand. Leaf litter samples were dried at 70 °C for three days in an oven with forced circulation of air (Riossa HCF-4848D, Mexico.). The net mass of nutrients returning to the soil was estimated from the dry weight of leaf litter and the concentration of nutrients (N, P, K, Ca and Mg).
On the other hand, 25 litterbags of 17 × 25 cm with an opening of 2 mm2 were placed on each stand (Bärlocher, 2005). These litterbags contained 20 g of previously dried litter. The sample did not include branches or fragments of wood. The bags were collected at different time intervals (63, 126, 217, 252 and 336 days) in such a way that the decomposition rate could be estimated according to the following exponential model (Schlesinger & Bernhardt, 2013):
where
Y |
remaining leaf litter mass (g) |
C |
initial leaf litter mass (g) |
k |
decomposition rate (g·day-1) |
t |
time (days) |
Sampling of bags was initially planned every 60 days, but finally it was made in accordance with the availability of resources for the study. The changes in the net mass of leaf litter during the year allowed us to estimate the rate of decomposition.
Reabsorption of nutrients
The reabsorption of N, P and K from the foliage was estimated with the difference in nutrient concentration between the fully developed leaves (August) and the senescent leaves (November) 2014. The total sample was 20 trees (10 per stand). The mass of N, P and K reabsorbed was obtained from the estimated reabsorption percentage. The reabsorption was obtained only for the most mobile macronutrients in the leaf, since the least mobile, especially Ca, cannot be estimated with the method used in this study.
Output of nutrients due to forest harvest
In order to have a conservative scenario, which included additional removal of trees for causes other than the forest harvesting plan, 4 % of the actual tree volume was taken as reference, such as the volume of wood extracted annually and its equivalent in mass of trunks and branches, regarding the density of the wood. This data was supported by information from the area management plan that estimates an annual current increase of 2 to 3 %, which is consistent with that reported by Zepeda and Acosta (2000) in forests of P. montezumae. The output of each nutrient was estimated with nutrient concentrations in trunks and branches. The mass of the foliage was not taken into account since it remains on the site and is not part of the outputs.
Annual requirement of nutrients and potential reserves
According to data from the forest management plan and the nutrient demand of other components such as roots, it was considered reasonable to maintain 4 % as an annual current increment in wood, just as it was done in the output of nutrients. Therefore, this reference helped to estimate the annual net gain of nutrients of wood components assuming the proportions of trunk, branches and leaves of the destructive sampling. The mass of N, P and K, required annually (kg·ha-1) for the formation of components, was calculated as indicated by Schlesinger and Bernhardt (2013). According to these authors, the uptake is the sum of the nutrients in biomass plus the returning to the forest floor, and the requirement is the sum of uptake and reabsorption. Because the reabsorption amounts of Ca and Mg could not be estimated, the requirements for these nutrients were not calculated here.
Assuming that the main outputs of nutrients originate from the forest harvest and that the nutrient capital of the soil is that estimated in each profile, the number of years that the soil could provide nutrients per tree growth was calculated under the current conditions of forest harvest (Martiarena, Wallis, & Knebel, 2009). That is, the estimated available amount of each nutrient was divided by the annual requirement of the stand.
Statistical analysis
A comparison of means of the dasometric data, leaf litter decomposition rate, soil nutrient mass and forest floor between the two stands was made using the Student T test (P = 0.05). The stand-level information on requirement, uptake and reabsorption of nutrients was not compared with this method, since only an average value per stand is generated.
Results and discussion
The comparison of dasometric data, leaf litter decomposition rate, soil nutrient mass and forest floor between the two stands did not indicate statistical differences (t, P > 0.05), for this reason, the discussion of the study focused mainly on the overall analysis, more than in comparison of stands. Although the statistical strategy of three repetitions per stand in this study is limited, it was considered important to show and discuss the data from an overview, given the importance of promoting studies for the evaluation of sustainability in forest areas under commercial harvesting in Mexico.
Dasometric data and nutrients in biomass
Stand I had a density of 180 trees·ha-1 with average values of diameter at breast height (DBH) of 39.5 cm, total height of 25.3 m, age of 86.3 years and tree volume of 269 m3·ha-1. Stand II had a density of 125 trees·ha-1, DBH of 44 cm, total height of 24.0 m, age of 90.3 years and tree volume of 224 m3·ha-1.
Stand I and II had total aboveground biomass of 133 and 120 Mg·ha-1, respectively, values expected for pine forests in central Mexico (Mendoza-Ponce & Galicia, 2010). The results of the tree destructive sample showed that the total biomass was distributed as follows: 89.5 (± 0.8), 7.5 (± 0.7) and 3 (± 0.4) for trunk, branches and leaves, respectively. These values, except for foliage, are different from those reported by Keyes, Zárate, Martínez, and Garcidueñas (1988) for the species studied; in the case of the trunk, the biomass was 8 % higher, and in the case of branches, it was 60 % lower. The mass of N was distributed in the following order trunk, foliage and branches (Table 1), which is the pattern observed in other Pinus species (Goya et al., 2003; Merino, Rey, Brañas, & Rodríguez-Soalleiro, 2003), while the mass of Ca and Mg was concentrated in the trunk and with similar amounts in branches and leaves.
Table 1 Nutrient mass per tree component in two stands of Pinus montezumae Lamb. in Nanacamilpa, Tlaxcala.
| Stand | Components | N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|
| (kg·ha-1) | ||||||
| Stand I | Trunk | 156.7 | 4.5 | 7.3 | 148.3 | 101.5 |
| Branches | 29.0 | 1.8 | 7.5 | 9.4 | 8.3 | |
| Foliage | 56.2 | 6.1 | 13.1 | 8.94 | 8.0 | |
| Total | 241.9 | 12.4 | 27.9 | 166.7 | 117.7 | |
| Stand II | Trunk | 141.6 | 4.1 | 6.6 | 134.0 | 91.7 |
| Branches | 26.2 | 1.6 | 6.8 | 8.5 | 7.5 | |
| Foliage | 50.8 | 5.5 | 11.8 | 8.1 | 7.2 | |
| Total | 218.6 | 11.2 | 25.3 | 150.6 | 106.4 | |
| CV (%) | Trunk | 6.0 | 45.1 | 32.4 | 7.7 | 13.5 |
| Branches | 17.4 | 54.4 | 29.5 | 13.8 | 42.8 | |
| Foliage | 5.9 | 16.3 | 33.4 | 26.9 | 24.9 | |
No statistically significant differences were found between the stands (t, P > 0.05). CV = coefficient of variation of determinations in the laboratory.
Nutrients of forest floor
Biomass of forest floor in stands I and II was 22.2 and 21.5 Mg·ha-1, respectively. These values are lower than those reported by Merino et al. (2003) for Pinus radiata D. Don., and by Garcidueñas (1987) for P. montezumae. The difference in litter biomass could be related to the age of the stands and tree density, since the studies cited were carried out in younger stands with higher density. Although no data of the same species were found for comparison purposes, the results show values expected for pine communities; for example, in old forests of Pinus hartwegii Lindl. from the center of Mexico, 18.7 Mg·ha-1 have been estimated in the forest floor (Mendoza-Ponce & Galicia, 2010).
The nutrient mass of the forest floor in stand I was 150.2, 9.6, 7.9, 95.2 and 40.6 kg·ha-1 de N, P, K, Ca and Mg, respectively; and in stand II, in the same order, it was 173.6, 9.4, 8.3, 91.6 and 34.4 kg·ha-1. In this regard, Garcidueñas (1987) reported higher values in the nutrient mass of the forest floor, because the concentration of nutrients in litter was higher. Another possible reason may be the rate of decomposition of leaf litter. Although the average annual precipitation in the study area mentioned was greater (> 1 000 mm), average annual temperature was 6 °C lower than that of this study (8 °C); according to Landsberg and Gower (1997), low temperatures limit decomposition. In a 42 years old-plantation of Pinus patula Schltdl. & Cham., the main reservoir of nutrients was the forest floor; this highlights the importance of leaf litter as a reservoir of nutrients in soil (Dames, Scholes, & Straker, 2002).
Mineral soil
Four horizons were identified in the soil profile of each stand (A, AB, Bw and Bt). Soil depths to the C horizon and the mass of nutrients in mineral soil are shown in Table 2. The amount in mass of nutrients is influenced by the thickness of horizons and bulk density that increased with the depth of the soil of 0.68 to 1.07 Mg·m-3. The total nutrient estimates, at a depth of 80 cm, were similar for both stands with average values of 5 419, 68, 1 245, 9 163 and 2 029 kg·ha-1 of N, P, K, Ca and Mg, respectively (Table 2).
Table 2 Nutrient mass in mineral soil of two stands of Pinus montezumae Lamb. in Nanacamilpa, Tlaxcala.
| Stand | Horizons | N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|
| (kg·ha-1) | ||||||
| I | A (0-17 cm) | 1 693.4 | 7.0 | 179.4 | 1 818.3 | 279.9 |
| (12.0) | (54.6) | (5.26) | (15.5) | (13.4) | ||
| AB (17-40 cm) | 2 280.4 | 9.1 | 289.6 | 2 506.9 | 488.2 | |
| (35.2) | (91.1) | (8.8) | (10.3) | (40.6) | ||
| Bw (40-70 cm) | 1 026.5 | 41.7 | 521.9 | 3 393.1 | 856.7 | |
| (30.2) | (25.3) | (15.1) | (13.4) | (35.1) | ||
| Bt (70-115 cm) | 398.8 | 8.9 | 258.5 | 1 353.1 | 400.1 | |
| - | - | - | - | - | ||
| Total estimated at 80 cm | 5 399.1 | 66.7 | 1249.4 | 9 071.4 | 2 024.9 | |
| II | A (0-15 cm) | 1 734.4 | 10.4 | 171.6 | 2 002.1 | 289.1 |
| (35.7) | (19.3) | (17.7) | (2.3) | (9.12) | ||
| AB (15-32 cm) | 1 549.5 | 3.1 | 93.9 | 1 024.4 | 200.3 | |
| (15.8) | (69.3) | (19.6) | (21.5) | (9.21) | ||
| Bw (32-63 cm) | 1 691.1 | 60.4 | 110.0 | 2 493.8 | 710.7 | |
| (28.4) | (74.6) | (58.0) | (16.7) | (25.4) | ||
| Bt (63-95 cm) | 662.4 | 20.9 | 278.9 | 1 874.5 | 741.3 | |
| - | - | - | - | - | ||
| Total estimated at 80 cm | 5 440.1 | 70.1 | 1241.6 | 9 255.2 | 2 034.1 | |
| Differences between stands (%) | (0.76) | (5.10) | (0.62) | (2.03) | (0.45) | |
The estimates for Bt horizon were fitted to the depth of 80 cm. Coefficient of variation between parentheses (%); the measurement of Bt horizon had no replication.
Leaf litter fall and descomposition
Figure 1 shows the leaf litter production in two stands of P. montezumae. The production in both stands was similar with 6.3 6.3 Mg ha-1 year-1, with a maximum in March (1.3 Mg·ha-1) that corresponds to the driest season. Rodríguez-Sanfuentes and Álvarez-Muñoz (2010) mention that the most common factor that determines abscission of leaves is the response of trees to water stress due to drought seasons. In September there was another increase in leaf litter production, which coincided with the end of the rainy season. This second peak of production in autumn is also shown by Pseudotsuga menziesii (Mirb.) Franco (Portillo-Estrada et al., 2013). The results of this study are in the range reported for other Pinus species ranging from 3.9 to 7.8 Mg·ha-1·year-1 (León, González, & Gallardo, 2011; Luna & Hernández, 2009; Ramírez-Correa, Zapata-Duque, León-Peláez, & González-Hernández, 2007). Pinus sylvestris L. and P. menziesii forests in Europe produce 2.86 and 4.34 Mg·ha-1·year-1 of litter (Portillo-Estrada et al., 2013). The amount of nutrients returning to the soil was estimated at 49.4, 2.7, 2.2, 21.4 and 8.4 kg·ha-1·year-1 of N, P, K, Ca and Mg, respectively. The amount of P that returned to the soil was low; in this regard, it has been reported that this element has low mobility in both natural and intensive systems; for example, in plantations of P. radiata 3.5 kg·ha-1·year-1 returns to the soil via leaf litter (Rivaie, 2014). Although the amount of K is also scarce, there is usually a return of this element to the soil through crown and trunk washing (Binkley & Fisher, 2013), which was not done in this study.
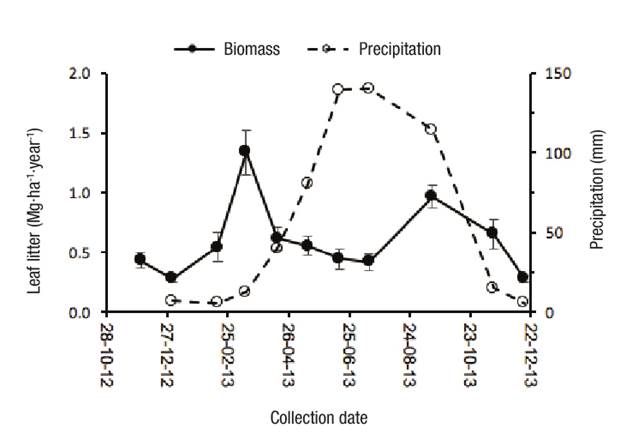
Figure 1 Average annual leaf litter production in the period 2012-2013 in two stands of Pinus montezumae in Nanacamilpa, Tlaxcala. The vertical lines represent the standard error of the mean.
Leaf litter decomposition rates were -0.33 and -0.34 for stand I and II, respectively. These values are those expected for temperate forests (-0.14 to -0.69) (Landsberg & Gower, 1997). León et al. (2011) reported a decomposition rate of -0.5 for P. patula in sites with 2 000 mm of precipitation, while Rocha-Loredo and Ramirez-Marcial (2009) reported decomposition rates greater than -0.5 in pine-oak associations in southern Mexico, where the average annual temperature varies from 14 to 25 °C.
Reabsorption of nutrients
N reabsorbed was 39 % in stand I and 30 % in stand II, for P it was 36 % in stand I and 40 % in stand II, and for K it was 51 % in stand I and 53 % in stand II. The mass of N, P and K reabsorbed in stand I was 21.9, 2.2 and 6.7 kg·ha-1·year-1, respectively, and in stand II it was 15.2, 2.2 and 6.2 kg·ha-1·year-1. N and P values are similar and those of K are 40 % lower than those found in other Pinus species (Dames et al., 2002; Martín, Santa Regina, & Gallardo, 1996; Peláez, Hernández, & Lancho, 2008; Pérez, Goya, Bianchini, Frangi, & Fernández, 2006). Compared with Garcidueñas (1987), who studied P. montezumae, the reabsorption values of N and P are between 1.7 and 2 times lower. The previous result could be because retranslocation is greater in younger stands than in old stands such as the one studied. Dames et al. (2002) suggested that the limitation of P in soil is compensated by the resorption rate (40 % value found in this study); perhaps in response to the low transfer of this nutrient from the forest floor to the mineral soil (Landsberg & Gower, 1997). The high reabsorption of K may be associated with high mobility and not with availability in the soil (Martín et al., 1996).
Output of nutrients due to forest harvesting
The output of nutrients due to forest harvesting is shown in Table 3. The biomass exported from the forest due to forest harvesting is 5.17 Mg·ha-1·year-1 in stand I and 4.29 Mg·ha-1·year-1 in stand II. These values are lower than those reported in 20 years-Pinus taeda L. plantations, under conservation practice, harvesting only trunks and retention of the rest of residues (Goya et al., 2003; Martiarena et al., 2009). The percentage of nutrients extracted by harvest, with respect to the magnitude of the reservoir in aboveground biomass is low, does not exceed 4.2 %; regarding the reservoir of the forest floor, the outputs represent 3 % for P and 13 % for Mg. However, the problem of conserving forest productivity lies in promoting practices that stimulate the mineralization of organic material, to ensure the provision of nutrients, especially when recycling P (Maynard et al., 2014).
Table 3 Average mass of nutrients extracted annually by forest harvesting in two stands of Pinus montezumae Lamb. in the ejido of Nanacamilpa, Tlaxcala.
| Stand | Components | Biomass | N | P | K | Ca | Mg |
|---|---|---|---|---|---|---|---|
| kg·ha-1·year-1 | |||||||
| I | Trunk | 3 909 | 5.1 | 0.15 | 0.24 | 4.9 | 3.3 |
| Branches | 386 | 1.1 | 0.07 | 0.29 | 0.4 | 0.3 | |
| Total | 4 296 | 6.3 | 0.22 | 0.53 | 5.2 | 3.6 | |
| II | Trunk | 4 708 | 6.2 | 0.18 | 0.29 | 5.9 | 4.0 |
| Branches | 465 | 1.4 | 0.08 | 0.35 | 0.4 | 0.4 | |
| Total | 5 174 | 7.5 | 0.26 | 0.64 | 6.3 | 4.4 | |
Requirement of nutrients and potential reserves
The average annual growth expressed in biomass was 5.3 and 4.8 Mg·ha-1, in stands I and II, respectively. These values are slightly lower than the one reported by Garcidueñas (1987) for the same species, perhaps because this study was carried out in a plantation where silvicultural practices were more controlled, which resulted on faster tree growth rates. Based on the determinations of the present study, the amounts of N, P, K that trees require for its growth are from 73 to 81, 5.2 to 5.3 and 9.4 to 10 kg·ha-1·año-1, respectively (Table 4). Taking into account that the potentially mineralizable nitrogen is approximately 3 % of the total nitrogen (Schlesinger & Bernhardt, 2013), the results indicate that 5 399 kg of N estimated in this study represent 162 kg of mineralizable N; that is, the soil could supply up to twice the annual requirements of the stand. On the other hand, the results highlight the importance of maintaining the organic surface material of soil and suggest some more intensive silvicultural practices, such as strong and early thinning to expose leaf litter and accelerate its decomposition.
Table 4 Uptake and anual requirement of nutrients in two stands of Pinus montezumae Lamb. in the ejido of Nanacamilpa, Tlaxcala.
| Stand | Nutrient | ANP | RFF | Uptake | Reabsoption | Requirement |
|---|---|---|---|---|---|---|
| (kg·ha-1·year-1) | ||||||
| I | N | 9.7 | 49.5 | 59.2 | 21.9 | 81.1 |
| P | 0.5 | 2.6 | 3.1 | 2.2 | 5.3 | |
| K | 1.1 | 2.2 | 3.3 | 6.7 | 10.0 | |
| II | N | 8.7 | 49.5 | 58.2 | 15.3 | 73.5 |
| P | 0.4 | 2.6 | 3.0 | 2.2 | 5.2 | |
| K | 1.01 | 2.2 | 3.2 | 6.2 | 9.4 | |
ANP: anual net profit, RFF: return to the forest floor
Under current conditions, the time that soil nutrients could sustain tree growth varies between 256 and 1 952 years. K is the nutrient that could maintain the system longer, between 1 234 to 1 952 years; followed by Ca, between 1 422 to 1 439 years; N between 706 to 909 years; Mg between 458 to 539 years; while for P it is from 256 to 430 years. According to the results, the current harvesting conditions can be considered as sustainable, because the nutrients evaluated could maintain the growth of the vegetation for 256 years. Even though this estimate is based on a simplification, it is useful as a preliminary reference, given the lack of information for forests harvested in Mexico. However, the sustainable term is actually not very tight, since each specialist interprets it in a different way. Strictly speaking, a sustainable system maintains productivity permanently. This concept does not apply to a forest system, since tree communities are subject to change. A time scale to define long-term sustainability in a forest system is estimated from 150 to 200 years (Patzek & Pimentel, 2005). Therefore, the results indicate that, if there is no adequate management, P is the most likely element that could affect the sustainability of the forest studied.
Conclusions
The amount of soil nutrients in Pinus montezumae stands exceeded the amount of nutrients in the aboveground biomass. Leaf litter decomposition rates of -0.33 and -0.34 are those expected for temperate ecosystems. These rates indicate that the litter takes an average of three years to decompose, assuming steady conditions of this reservoir. The nutrient reserves in forest floor and mineral soil are enough for the intensity of current forest harvesting. The potentially mineralizable nitrogen was estimated twice in the requirements of the tree mass. The transfer of litter to forest floor and the high reabsorption of macro-nutrients could satisfy the nutritional requirements of the stands studied. If current conditions are maintained, the management of P. montezumae stands could continue for more than two centuries, but if sustainable harvest practices are no longer used, phosphorus would be the first element that would put such sustainability at risk. In spite of being a limited study in stands and replications, the information generated by this study is relevant and it is expected that it may promote the approach of other studies to evaluate the sustainability of forest harvesting in Mexico.











 text in
text in 


