Introduction
Acrocomia aculeata (Jacq.) Lodd. ex Mart., known in Paraguay as mbokaja, belongs to the family Arecaceae and is native to tropical forests (Bandeira, 2008). This species is widely distributed throughout the tropical and subtropical regions of the Americas, from southern Mexico and the Antilles, to the southern region, including Brazil, Argentina and Paraguay (Sorol, Haupenthal, & Reckziegel, 2012). In Paraguay, A. aculeata is naturally abundant in the eastern region and in some areas of the Chaco.
The species A. aculeata is is a heliophilous palm that lives in open areas with high solar radiation. It adapts to sandy soils (60 to 75 % sand) with a low level of water, although it prefers deep and well-drained ones; it can also be found in soils derived from sandstones and basalts. The palm does not grow in low soils of a hydromorphic nature that are heavy and poorly drained (Lorenzi, 2006).
Acrocomia aculeata has a stipe that can range between 10 and 15 m in height and between 20 and 30 cm in diameter. This stem has a covering of dark-colored spines, about 10 cm long (Lorenzi, 2008). The leaves are green, pinnate and alternate, 4 to 5 m long and are arranged in different planes, giving the crown a feather crown appearance. The leaves have approximately 130 leaflets, which are 30 to 60 cm long, 1 to 2 cm wide and glossy green on each side. The underside is light green with thorns in the central region (Lorenzi, 2006).
The inflorescences appear between the leaves; they are spadix-shaped, measure 80 to 130 cm in length, are decurrent and are covered by a bract. When the inflorescence reaches maturity it becomes turgid, with a location perpendicular to the stem. Henderson, Galeano, and Bernal (1995) indicate that the flowers are unisexual, with the female flowers appearing at the base of the inflorescence and male ones at the top.
Regarding fructification, Lorenzi (2008) reports that it occurs throughout the year and that the fruits mature between September and June. The fruit is a globose drupe with a diameter between 2.5 to 5 cm, constituted by a brittle pericarp at maturity and a fibrous, mucilaginous, sugary mesocarp rich in yellow-orange glycerides. The endocarp is strongly attached to the mesocarp; it has a bony structure, is dark in color, has a thickness of approximately 3 mm and contains the edible oily seed (Bandeira, 2008).
The seeds of A. aculeata are orthodox and have slow germination and dormancy; they are formed by an endosperm where the embryo is immersed, usually at one end. The embryo, conical and small, is rich in nutritive material and is enveloped by a mass of corneal tissue or albumen. Knowledge of the seed’s morphology is essential for the analysis of the vegetative cycle and the study of regeneration of the species. From the morpho-anatomical study of the seeds and their embryos, information on germination, storage and viability can also be obtained (Moura et al., 2008).
Oil is obtained from the fruits, both from the drupe and the internal nut, and is used to produce biodiesel. The oil is also consumed by local populations, due to its high nutritional value. In Paraguay, intensive cultivation of A. aculeata is being implemented, aimed at covering the demand for oil obtained from its fruits (Instituto Interamericano de Cooperación para la Agricultura [IICA], 2007). According to IICA (2007), oil production reaches up to 4 000 L·ha-1, with higher yield than other oil crops such as avocado (Persea americana Mill.), castor bean (Ricinus communis L.), rapeseed (Brassica napus L.), peanut (Arachis hypogaea L.), sunflower (Helianthus annuus L.), tung (Aleurites fordii Hemsl.) and soybean (Glicine max L.). Paraquay has an estimated 6 million mbokaja plants, suggesting an annual production of about 170,000 t of fruit (Souto, 2008). According to Silva (2006), A. aculeata can become one of the most important palm oils commercially, given the variety of products derived from the fruits (20 to 30 % oil, 5 % edible flour, 35 % forage and 35 % high calorific value fuel).
In this regard, this paper aims to describe the morphoanatomy of the A. aculeata embryo at the cellular level.
Materials and methods
Plant material
The embryos were obtained from mature fruits of A. aculeata, harvested between February and March 2014. The fruits were collected in 10 individuals from a native population, near the city of San Lorenzo, Central Department (25° 21' 54.80" S and 57° 30' 8.77" W) in Paraguay. Twenty fruits were collected per bunch from each mother plant, making a total of 100 fruits per individual.
The seeds were mixed and dried at room temperature for 20 days, facilitating the removal of the pericarp. Once removed, the endocarp was broken with the aid of a manual press and the seeds containing the zygotic embryos were obtained. Seeds with symptoms of fungal infections, bacterial infections or insect attacks were removed.
Morpho-anatomical study
Fixation, dehydration and inclusion of embryos in resin blocks
The embryos were carefully extracted from the seeds with a scalpel, for the embedding process in resin blocks. Prior to this process, the samples were fixed for 24 h with a Karnovsky solution (5 % glutaraldehyde and 4 % paraformaldehyde in 0.2 M cacodylate) and preserved with 0.025 M cacodylate. For this, the samples were deposited in 2-mL microcentrifuge tubes containing 1 mL of Karnovsky's solution. Subsequently, the support with the open tubes was placed in a vacuum hood for periods of 1 min, to facilitate the penetration of the fixative. Finally, the tubes were cooled to 4 °C for 4 h and stored at 4 °C under dark conditions (Pérez‐de‐Luque et al., 2006).
The samples were dehydrated using a series of ethanol/water mixtures (50, 80 and 95 %), for 12 h; subsequently, they were stored at 4 °C under dark conditions. Histresin molds were filled with infiltration solution (dimethyl sulfoxide), hardener (barbituric acid derivative) and paraffin; samples were placed in the position of interest. The resin blocks were removed from the molds and left to dry at room temperature. Two samples were placed in each block, one in a transverse position and the other in a longitudinal position, with the aim of having section cuts of both types.
Once the blocks were removed from the molds, they were trimmed with a blade. Subsequently, 2-μm-thick cuts were obtained in the section of interest using a LEICA RM2245 microtome (Leica Mycrosistems GmbH, Wetzlar, Germany) with Spikker Specials carbon-tungsten blades.
Microscopy
The slides were placed in a 0.1 % TBO (Toluidine Blue-O) solution for 12 h. The 2-μm-thick sections were deposited on glass slides which were spread on a polylysine-coated slide (Thermo Scientific, Gerhard Menzel GmbH, Braunschweig, Germany) for a better fixation of the plant material to the surface. Each of the sections was rehydrated with deionized water.
The sections were dewaxed with xylene (Pérez‐de‐Luque et al., 2006) and stained with a 0.05 % TBO solution (Cahill, Benett, & McComb, 1992) in citrate buffer at pH 5 (Ruzin, 1999). The completely-dried samples were placed on slides with synthetic resin for quick mounting (Entellan®, Merck KGaA, Darmstadt, Germany).
The sections were analyzed qualitatively through a NIKON Eclipse 50i optical microscope (Nikon Instruments Inc., Melville-NY, USA), with interchangeable lenses (10x, 20x, 40x, 100x) that provided up to 1,000x magnification. Images of 2 560 x 1 920 pixels, taken with Nikon Instruments Inc. Plan Fluor series lenses, were obtained through a Nikon DS-Fi1 digital optical device (Nikon Instruments Inc., Melville-NY, USA) coupled to the microscope and connected to a computer via the Nikon DS-U2 control unit (Nikon Instruments Inc., Melville-NY, USA).
Results and discussion
Morpho-anatomical characterization of the A. aculeate embryo
The A. aculeata embryo is small, greenish-yellow with a lanceolate shape. It has a proximal region, located peripherally to the seed, which corresponds to the petiole of the cotyledon, and a distal region, in the form of a protuberance, in the limulus or cotyledon haustorium. The embryo’s structure has the general pattern observed in other palm species, with a protuberance and pronounced invaginations of the haustorium. Figure 1 shows the longitudinal section of the A. aculeate embryo, in which three regions are distinguished: cotyledon limbus, constriction and cotyledon petiole.

Figure 1 Longitudinal section of the Acrocomia aculeata embryo. The cotyledonary limbus (cl), the constriction zone (c) and the cotyledonary petiole (cp) are observed.
The cotyledon petiole averages 4.5 mm in length and 1.5 mm in diameter; it is formed by an external protodermis, a fundamental meristem and procambial strands. The embryonic axis is inserted into a cavity in the proximal third of the cotyledon petiole and is aligned obliquely to the rest of the embryo. Figure 2 shows the primary meristematic, fundamental meristem and procambium tissues.

Figure 2 Primary meristematic (PM), fundamental meristem (FM) and procambium (Prc) tissues in the longitudinal section of the Acrocomia aculeata embryo.
In the transversal and longitudinal sections of the embryos, the well-differentiated plumule was observed, situated at an angle with respect to the longitudinal axis of the embryo, opposite to which is the meristematic zone, where the radicle will develop. The plumule consists of two leaf primordia and one apex, with the outline of a third primordium (Figure 3). All these structures are enclosed by the coleoptile.
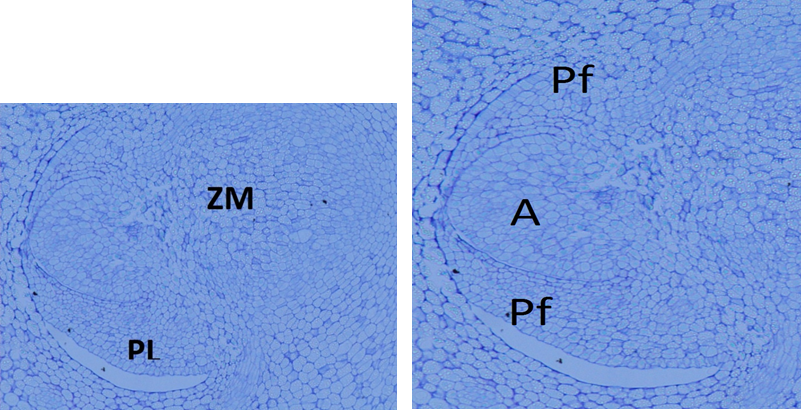
Figure 3 Longitudinal section of an Acrocomia aculeata embryo, where the plumule (PL) and the radical meristematic zone (MZ) are observed. Inside the plumule are the apex (A) and the leaf primordia (Lp).
Externally, the embryo has a longitudinal slit or cotyledonary window, at the level of the seedling; the layer of protodermal cells covering said window continues with that of the coleoptile. Figure 4 shows the cross-section of an embryo, showing the cotyledon window that will allow the emergence of the seedling (radicule and plumule).
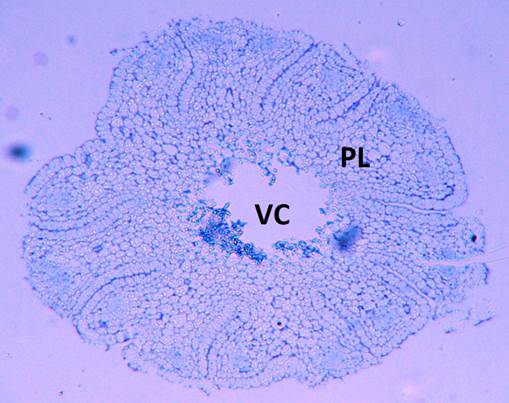
Figure 4 Cross-section of Acrocomia aculeata embryo, at the level of the cotyledonary petiole, showing the cotyledonary window (cw) that will allow the emergence of the seedling (SE).
According to Cabello (2008), the seedling phase begins with the reactivation of the embryo and its emergence from the seed, through the emission of the radicle and the plumule. When germination occurs, the embryo is elongated by detaching the operculum, from which the cotyledonary or ligule pod emerges to the exterior, through the germinative pore. Subsequently, the ligule is included, splits and the radicle and the plumule arise. The haustorium develops from the cotyledonary limb of the embryo, occupying the internal cavity of the seed. The haustorium has an invaginated surface, with an epidermis formed by rectangular cells in radial direction, with very dense cytoplasm and prominent nuclei. The subepidermal layer, monostratified, presents cells of regular shape and size. Towards the interior, the parenchyma cells are irregular and gradually increase in size, leaving large intercellular spaces.
In palms, according to Tomlinson (1990), the embryo is always tiny in relation to the total size of the seed and the endosperm. The A. aculeata embryo is no exception and the observations made in this work confirm the claims of Cabello (2008), who indicates that the embryo of the palms is incompletely developed. This agrees with what has been stated by Nikolaeva (1969), who points out that the individuals of the Arecaceae group present endogenous morphological dormancy, since they have an embryo that has not reached its morphological development at the time of seed maturation.
In general, the A. aculeata embryo is similar to that of other palms of the same group, in terms of shape and inclusion in the endosperm. The endosperm arrangement of Cocos nucifera L. and Jubebaea chilensis (Molina) is similar, as is the location of the embryo under the germinative pore (Heathcock & Chapman, 1983; Sugimura & Murakami, 1990) in its cylindrical shape. Most of the tissue of the embryo corresponds to the cotyledon with the presence of a small constriction that divides it into two parts. The same goes for the embryo of Elaeis guineensis fo. dura Becc., which is also cylindrical-conical (Quero, 1992). The embryo of Acrocomia mexicana Karwinky ex Mart. is opposite to one of the poles, while Bactris mexicana Martius, another species of the Arecaceae group, can be basal or dorsal (Quero, 1992).
The embryos of A. aculeata consist of protoderm, plumule and well-defined procambal strands (Moura et al., 2008). The protoderm shows invaginations and, below, cells of the fundamental meristem. Starch grains accumulate in the cells of the fundamental meristem under intense division (Moura et al., 2008), especially in those with less dense cytoplasm. In the case of A. aculeata, a cotyledonary window is located in the longitudinal direction of the embryo, which simulates a tangential cut eyelet, similar to that of E. guineensis (Vallade, 1966a). Acrocomia aculeata, like E. guineensis fo. dura and J. chilensis, presents the embryo plumule in lateral position and is well differentiated into two leaves and an apex, which bears on its flanks the outline of the third leaf. This plumule is completely enclosed in a fold of the cotyledon, whose lips are completely visible at the level of the cotyledonary opening. The root is not individualized; in the fundamental parenchyma, there is a region of radicular aptitude that will participate in the formation of the root, at the time of germination (Vallade, 1966a).
The epidermis of the anatomical structures of A. aculeata has a layer composed of thin-walled elongated cubic cells, presenting meristematic cells of globose appearance in the external region of the mesophyll, interspersed with bundles of radially elongated cells. The middle region of the mesophile is predominantly composed of parenchymal cells (Reis, Mercadante-Simões, & Ribeiro, 2012).
Some cells of the inner part of the A. aculeata embryos are larger than those of the periphery with dense cytoplasm; in general, cell divisions occur in the distal region both in the periphery and in the interior. These cell divisions occur mainly in the fundamental meristem, near the procambial strands. In these tissues, groups of cells that present a wall with apparent thickening and mucilaginous appearance are concentrated. The apparent thickening may be the result of the disintegrated cell wall, pectin storage and mucilage formation (Moura et al., 2008).
Conclusions
In general, the Acrocomia aculeata embryo is similar to that of other palms of the same group, in terms of shape and inclusion in the endosperm. Morphoanatomical studies can help to improve the description of processes that lead to a tissue reacquiring its embryogenic potential, which can be applied to the embryogenesis of A. aculeata. The morphoanatomical study could also be used to approach germination trials from a new perspective, in order to find treatments that overcome the dormancy in the seeds of this palm.











 texto en
texto en 


