Introduction
Ectomycorrhiza is a mutualistic symbiosis of great ecological importance that is established between more than 20 000 species of fungi and the roots of more than 5 000 species of angiosperm and gymnosperm plants (Pérez-Moreno, Lorenzana, Carrasco, & Yescas-Pérez, 2010; Rinaldi, Comandini, & Kuyper, 2008). The main benefit of ectomycorrhizal symbiosis for plants is nutrient supply and protection against stress factors, while for fungi it is the provision of carbon by host plants (Smith & Read, 2008). In addition to accessing soil nutrients, ectomycorrhizal fungi and the mycorrhizosphere provide a unique niche for other microorganisms, including a vast community of bacteria. Due to their beneficial properties, various groups of rhizospheric bacteria are known as mycorrhiza helper bacteria (MHB), which are characterized by stimulating the development and establishment of mycorrhizae (Cumming, Zawaski, Desai, & Collart, 2015).
Currently, at international level, one of the criteria for selecting ectomycorrhizal fungi is their edibility, which makes them into another useful forest environment resource. Although there is potential for coinoculation with edible ectomycorrhizal fungi and bacteria (Sousa, Franco, Ramos, Oliveira, & Castro, 2015), information on its effect on the physiology, nutrition and growth of important forest plants is scarce. On the other hand, in Mexico, deforestation is an issue of great importance; from 2005 to 2010, 775 000 ha of forests and jungles were lost in the country (Food and Agriculture Organization of the United Nations [FAO], 2010). In addition to this problem, the survival of pines transplanted from the greenhouse to the field is highly variable and at some sites may be 0 % (Cetina, 2004).
Several species of Pinus are used in reforestation programs; however, their establishment has failed because in this process and during their growth they are dependent on ectomycorrhiza, which is not traditionally incorporated into plant production in Mexico (Pérez-Moreno, 2016; Valdés, Ambriz, Camacho, & Fierros, 2010). The aim of the present study was to evaluate the effect of the inoculation of an edible ectomycorrhizal fungus and two mycorrhiza helper bacteria on the growth, physiology and nutrient content of Pinus montezumae Lamb.
Materials and methods
The P. montezumae germplasm used comes from San Rafael, municipality of Tlalmanalco, State of Mexico. The seeds were sterilized with 30 % H2O2 for 20 min. The inoculum was prepared with spores from a pine forest in the municipality of Ozumba, State of Mexico. The inoculum was obtained from Hebeloma mesophaeum (Pers.) Quél. pilei, which were cut from the stipes and dehydrated at 35 °C (Jersa® tray-type dehydrator, Mexico). Afterwards, the pilei were ground in an electric mill (Thomas Scientific®, Model 4 Wiley Mill, USA) through a 1-mm aperture sieve to obtain a homogeneous particle size. The inoculum was stored at 5 °C.
Substrate preparation and inoculation
The substrate consisted of a mixture of sand, bark and forest soil at a 2:2:1 ratio, which was sterilized three times with steam at a pressure of 1.3 kg · cm-2 at 125 °C for 5 h. The seeds were sown in 125 cm3 black plastic tubes containing the described substrate. Each plant was inoculated with a concentration of 107 to 108 spores of H. mesophaeum, determined with a hematocytometer. Two spore inoculations of the fungus were made: the first simultaneously with the sowing and the second 90 days later.
Cultivation of bacterial strains
The bacterial strain Cohnella sp., isolated from the P. montezumae root, was provided by the Colegio de Postgraduados Microbiology Laboratory, and Azospirillum brasilense Terrand Krieg et Dobereiner was provided by the Soil Microbiology Laboratory of the Center for Microbiological Science Research, part of the Science Institute of Benemérita Universidad Autónoma de Puebla. Both species were previously identified with molecular techniques in these laboratories. The strains were cultured in Merck® nutrient broth and kept in constant movement at 28 °C until reaching a concentration of 108 cfu-mL-1. The bacterial culture was centrifuged at 7 000 rpm and the supernatant was removed; the concentrate was washed with sterile distilled water to remove residues from the culture medium and resuspended in sterile distilled water to leave it at the same concentration of 108 ufc·mL-1. Subsequently, 10 days after the second inoculation with the ectomycorrhizal fungus, a single application of 3 mL of the bacterial suspension to each plant was made in the early hours of the day. The experiment was run for 420 days in a closed greenhouse without air filtration, temperature control or additional fertilization. The environmental conditions during the experiment were: mean annual temperature 20 °C (9 and 31 °C, minimum and maximum, respectively), average annual relative humidity 47 % (23 and 81 % minimum and maximum, respectively) and mean annual photoperiod of 11.9 h (10.9 and 13.1 h, minimum and maximum, respectively).
Variables evaluated
Diameter and dry weight
The stem diameter of seven randomly-selected plants per treatment was measured with a digital Vernier caliper at 30, 120 and 420 days after bacterial inoculation (dabi). Biomass was also evaluated in seven randomly-selected plants per treatment to determine root, shoot and total dry weight, dehydrating each component separately, for three days at 70 °C until constant weight.
Photosynthetic rate and content of chlorophylls and carotenes
The photosynthetic rate was evaluated at 240, 300 and 360 dabi in three plants per treatment with an infrared gas analyzer (IRGA, PP-Systems® model Ciras-3, USA) and a gas assimilation chamber (PP-Systems®, original accessory of Ciras-3 CPY-4, USA) in a greenhouse. An evaluation was not carried out at the end of the experiment due to practical constraints related to weather conditions. The content of chlorophyll a, b, total and carotenes was determined by the acetone method (Zhang, 1986) and the concentration of chlorophylls and carotenes was obtained using the equations of Lichtenthaler (1987).
Nutrient analysis
Nutrients were analyzed in four plants per treatment; nitrogen (N) was determined by the semimicro-Kjeldahl method (Bremner, 1965), total phosphorus (P) according to the method of Allen, Grimshaw, Parkinson, and Quarmbym (1997), and potassium (K) by extraction with ammonium acetate by flame photometry. Mycorrhizal colonization was evaluated using the techniques proposed by Agerer (1994) and Martínez-Reyes et al. (2012). The confirmation of mycorrhizal roots was carried out using microphotography techniques to reveal the characteristic structures of ectomycorrhiza, namely the mantle, Hartig net and external mycelium, under an optical microscope (Leica®, DM1000, EUA).
Experimental design and statistical analysis
The experiment was designed in random blocks with six treatments: 1) Uninoculated plants; and plants inoculated with: 2) H. mesophaeum (Hm), 3) Cohnella sp. (C), 4) A. brasilense (Ab), 5) Hm + C, and 6) Hm + Ab. Each treatment had 21 replicates, so in total there were 126 experimental units, each constituted by one plant. The data of the evaluated variables were subjected to an analysis of variance and Tukey's range test (P = 0.05) with the statistical package (Statistical Analysis System [SAS Institute Inc.], 2009). The mycorrhizal colonization values, expressed as percentages, were transformed and the natural logarithm was used in the analyses of variance.
Results and discussion
Dry weight and stem diameter of P. montezumae
Table 1 contains information on the dry weight of P. montezumae plants under different inoculation treatments. The root, shoot and total dry weight values were higher in the plants inoculated with the ectomycorrhizal fungus, alone or coinoculated with either of the two bacteria, compared to the uninoculated plants. When inoculated exclusively with either of the two bacteria, dry weights were statistically similar (P = 0.05) to those of the uninoculated plants. In the case of root dry weight there was a synergistic effect when Hm + Ab was inoculated; in this treatment, the weight was up to 6.4 times greater compared to uninoculated plants.
Table 1 Dry weight of Pinus montezumae plants under different inoculation treatments with an edible ectomycorrhizal fungus and two plant growth promoting bacteria. Results at 420 days after bacterial inoculation*.
| Treatments | Dry weight (g) | ||
|---|---|---|---|
| Root | Shoot | Total | |
| Uninoculated plants | 0.22 ± 0.02 c | 0.26 ± 0.02 c | 0.48 ± 0.04 c |
| Hebeloma mesophaeum (Hm) | 1.10 ± 0.07 b | 1.15 ± 0.12 ba | 2.25 ± 0.19 ba |
| Cohnella sp. (C) | 0.38 ± 0.05 c | 0.34 ± 0.02 c | 0.70 ± 0.07 c |
| Azospirillum brasilense (Ab) | 0.50 ± 0.06 c | 0.42 ± 0.04 c | 0.92 ± 0.10 c |
| Hm + C | 1.08 ± 0.05 b | 0.96 ± 0.08 b | 2.04 ± 0.12 b |
| Hm + Ab | 1.41 ± 0.12 a | 1.33 ± 0.08 a | 2.75 ± 0.18 a |
*Bacterial inoculation was done 100 days after sowing of P. montezumae. Values with the same letter in the same column are the same according to the Tukey test (P = 0.05). ± Standard error of the mean (n = 7).
With respect to stem diameter, Figure 1 shows that this variable increased to a greater extent in plants inoculated with both Hm and in plants with Hm + C and Hm + Ab at 30, 120 and 420 dabi. Inoculation with either bacterium did not cause differences in stem diameter compared to uninoculated plants.
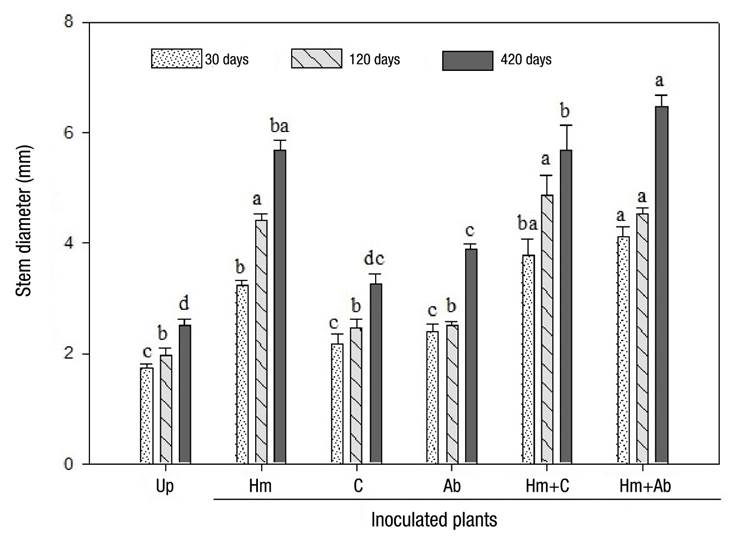
Figure 1 Stem diameter of Pinus montezumae plants under different inoculation treatments with an edible ectomycorrhizal fungus and two plant growth promoting bacteria. Up = uninoculated plants, Hm = Hebeloma mesophaeum, C = Cohnella sp., Ab = Azospirillum brasilense. The standard error of the mean (n = 7) is represented above the bars. Values with the same letter for each date are equal according to Tukey’s test (P = 0.05).
Growth stimulation, attributable to inoculation with ectomycorrhizal fungi, has been widely reported for the genus Pinus. Gómez-Romero, Lindig-Cisneros, and Del Val (2015) showed that Pinus pseudostrobus Lindl. increased shoot and root biomass when inoculated with Pisolithus tinctorius Pers. Perea-Estrada et al. (2009) recorded an increase in height, root dry weight and diameter of Pinus patula Schl. et Cham. and Pinus hartwegii Lindl. plants inoculated with Hebeloma spp., Laccaria spp. and Clavulina aff. cinerea. Similarly, Sebastiana, Tolentino, Alcantara, Salomé, and Bernardes (2013) reported that the dry weight of Quercus suber L. plants, inoculated with P. tinctorius, increased by 40 % compared to uninoculated plants. The present study is the first one that evaluates the effect of the coinoculation of ectomycorrhizal fungi and bacteria on the growth and physiology of P. montezumae. The synergism recorded due to the coinoculation of ectomycorrhizal fungi and bacteria has also been previously reported. For example, Kataoka and Futai (2009) reported increased root biomass in Pinus thumbergii Parl. coinoculated with Suillus granulatus L. and Burkholderia sp. On the other hand, Zhao, Xiao-Qin, Jian-Ren, Hao, and Gui-E (2014) showed that coinoculation with the bacterium Bacillus sp. and the fungi P. tinctorius and Lactarius insulsus (Fr.) Fr. (whose current valid name is L. zonarius [Bull.] Fr.) increased the growth and ectomycorrhizal colonization of Populus deltoides Marsh trees.
Photosynthetic rate of P. montezumae
According to Table 2, the differences in the photosynthetic rate of inoculated and uninoculated plants were observed from 300 dabi; the highest values were recorded in plants inoculated with Hm, Hm + C and Hm + Ab. The photosynthetic rate at 360 dabi was 3.7 and 4.0 times higher in plants inoculated with Hm + C and Hm + Ab, respectively, compared to uninoculated plants. As time elapsed, the photosynthetic rate increased in all treatments, particularly in Hm + Ab; in this case, the photosynthetic rate at 360 dabi was 2.6 times greater than at 240 days. At 300 and 360 dabi, no differences were observed between plants inoculated only with bacteria and those not inoculated. This is the first time that the photosynthetic rate in P. montezumae plants coinoculated with ectomycorrhizal fungi and bacteria has been evaluated.
Table 2 Photosynthetic rate in Pinus montezumae plants under different inoculation treatments with an edible ectomycorrhizal fungus and two plant growth promoting bacteria.
| Treatment | Photosynthetic rate (g CO2·m-2·h-1) | ||
|---|---|---|---|
| 240 dabi | 300 dabi | 360 dabi | |
| Uninoculated plants | 0.21 ± 0.14 a | 0.24 ± 0.05 c | 0.23 ± 0.07 c |
| Hebeloma mesophaeum (Hm) | 0.73 ± 0.07 a | 0.77 ± 0.06 ba | 0.79 ± 0.16 ba |
| Cohnella sp. (C) | 0.28 ± 0.09 a | 0.28 ± 0.06 c | 0.25 ± 0.06 c |
| Azospirillum brasilense (Ab) | 0.32 ± 0.03 a | 0.34 ± 0.05 bc | 0.40 ± 0.03 bc |
| Hm + C | 0.71 ± 0.01 a | 0.73 ± 0.07 ba | 0.84 ± 0.05 ba |
| Hm + Ab | 0.36 ± 0.04 a | 0.88 ± 0.07 a | 0.94 ± 0.06 a |
dabi: days after bacterial inoculation (performed 100 days after sowing of P. montezumae). Values with the same letter in the same column are the same according to the Tukey test (P = 0.05). ± Standard error of the mean (n = 3).
The increase in the photosynthetic rate due to inoculation exclusively with mycorrhizal fungi has already been reported; however, to date, the effect of coinoculation with bacteria had not been investigated. Canton et al. (2016) indicated that inoculation of P. tinctorius in E. grandis resulted in higher photosynthetic rates than in uninoculated plants. Xu et al. (2015) mentioned that the ectomycorrhizal fungus Laccaria bicolor Maire inoculated in Picea glauca (Moench) Voss not only increases the photosynthetic rate, but also causes greater transpiration.
Chlorophyll and carotene contents of P. montezumae
The content of chlorophylls a and b, total chlorophylls and carotenes was higher (P = 0.05) in inoculated plants, regardless of the treatment. Plants inoculated with Hm had a higher content of chlorophyll a compared to uninoculated plants, which did not occur in the case of chlorophyll b, total chlorophyll and carotene contents (Table 3). Yin, Deng, Chet, and Song (2014) indicated that the contents of chlorophylls a and b, and carotenes increased in Pinus sylvestris L. plants inoculated with S. luteus (L. ex Fr.) Gray.
Table 3 Chlorophyll and carotene content in Pinus montezumae plants under different inoculation treatments with an edible ectomycorrhizal fungus and two plant growth promoting bacteria. Results at 420 days after bacterial inoculation* .
| Treatments | Chlorophyll (mg·g-1) | Carotenes (μg·g-1) | ||
|---|---|---|---|---|
| a | b | Total | ||
| Uninoculated plants | 30.22 ± 1.59 b | 31.91 ± 1.23 b | 62.12 ± 2.50 b | 10.73 ± 0.53 b |
| Hebeloma mesophaeum (Hm) | 42.24 ± 2.84 a | 42.43 ± 4.15 ba | 84.65 ± 6.92 ba | 10.83 ± 0.60 b |
| Cohnella sp. (C) | 39.53 ± 2.49 ba | 50.27 ± 3.39 a | 89.77 ± 5.69 a | 11.85 ± 0.72 ba |
| Azospirillum brasilense (Ab) | 36.63 ± 2.24 ba | 44.22 ± 2.83 ba | 80.83 ± 4.99 ba | 11.75 ± 0.51 ba |
| Hm + C | 43.89 ± 3.61 a | 49.08 ± 4.75 a | 92.94 ± 8.26 a | 13.41 ± 0.88 a |
| Hm + Ab | 39.06 ± 2.13 ba | 39.89 ± 1.74 ba | 78.93 ± 3.80 ba | 13.41 ± 0.48 a |
*Bacterial inoculation was done 100 days after sowing of P. montezumae. Values with the same letter in the same column are the same according to the Tukey test (P = 0.05). ± Standard error of the mean (n = 7).
The carotene concentration recorded in the present study was higher when the bacteria were inoculated alone or in combination with the fungus Hm. This increase coincides with that reported by Mrnka, Tokárová, Vosátka, and Matejka (2009), who inoculated Hebeloma bryogenes Vesterh. and Cadophora finlandica C. J. K. Wang et H. E. Wilcox. in Picea abies L.
Mycorrhization of P. montezumae
Figure 2 shows the diagnostic structures of ectomycorrhiza. Treatments Hm, Hm + Ab and Hm + C produced the highest percentages of mycorrhizal live roots; 76.1, 76.4 and 68.7 %, respectively (Table 4).

Figure 2 General characteristics of the ectomycorrhizae of Hebeloma mesophaeum (Hm). a) Overview of Hm morphotypes showing abundant, external, whitish to cream mycelium; b) Morphotype of Hm with Azopirillum brasilense showing the external mycelium covering the entire ectomycorrhiza; c) Morphotype of Hm + Ab, from which the external mycelium has been removed; d) Cross section: Hartig net (rh), mantle (m) and external mycelium (me).
Table 4 Mycorrhizal colonization of Pinus montezumae plants under different inoculation treatments with an edible ectomycorrhizal fungus and two plant growth promoting bacteria. Results at 421 days after bacterial inoculation*.
| Treatments | Living roots (%) | Dead roots (%) | ||
|---|---|---|---|---|
| Mycorrhized | Non-mycorrhized | Mycorrhized | Non-mycorrhized | |
| Uninoculated plants | 2.75 ± 0.25 c | 84.60 ± 0.77 a | 1.88 ± 0.27 b | 10.74 ± 0.96 a |
| Hebeloma mesophaeum (Hm) | 76.15 ± 1.36 a | 8.62 ± 2.18 b | 8.73 ± 2.31 a | 6.47 ± 1.82 a |
| Cohnella sp. (C) | 12.89 ± 7.85 c | 66.81 ± 6.27 a | 1.82 ± 0.59 b | 18.35 ± 8.79 a |
| Azospirillum brasilense (Ab) | 12.95 ± 5.37 bc | 71.2 ± 5.95 a | 4.57 ± 0.98 ba | 11.27 ± 1.77 a |
| Hm + C | 68.67 ± 2.18 a | 10.85 ± 0.78 b | 10.52 ± 2.07 a | 9.94 ± 0.66 a |
| Hm + Ab | 76.41 ± 5.13 a | 5.02 ± 1.13 b | 10.82 ± 2.01 a | 7.70 ± 2.09 a |
*Bacterial inoculation was done 100 days after sowing of P. montezumae. Values with the same letter in the same column are the same according to the Tukey test (P = 0.05). ± Standard error of the mean (n = 3).
The mycorrhization values in this work are similar to those reported by Méndez-Neri, Pérez-Moreno, Quintero, Hernández, and Lara (2011), who determined 77 % colonization in Pinus greggii Engelm. inoculated with H. mesophaeum, Laccaria laccata Scop. and Suillus aff. pseudobrevipes A. H. Sm. These authors used the same spore concentrations and inoculation method as those employed in the present study. On the other hand, Sanchez-Zabala et al. (2013) recorded less than 30 % colonization in Pinus pinaster Aiton plants inoculated with Lactarius deliciosus L., Lactarius quieticolor Romagn., Pisolithus arhizus Scop. and S. luteus.
Content of N, P and K in P. montezumae
Table 5 shows the nutrient content in P. montezumae plants with the different inoculation treatments. When Hm was inoculated alone or coinoculated with either of the two bacteria, there was a greater amount of shoot, root and total N compared to uninoculated plants. When inoculated exclusively with either of the two bacteria, there were no differences in the amount of N, P and K compared to uninoculated plants, regardless of the part of the plant. The best treatment in the case of N was recorded in plants inoculated with Hm + Ab, whereas in the case of P and K it was constituted by the ectomycorrhizal fungus alone or coinoculated with either of the two bacteria. A synergistic effect of the Hm + Ab treatment on the amount of shoot and total N was observed.
Table 5 Nutrient content in Pinus montezumae plants under different inoculation treatments with an edible ectomycorrhizal fungus and two plant growth promoting bacteria. Results at 420 days after bacterial inoculation*.
| Nutrient | Uninoculated plants | Inoculated plants | ||||
|---|---|---|---|---|---|---|
| Hm | C | Ab | Hm + C | Hm + Ab | ||
| N (mg) | ||||||
| Shoot | 1.96 ± 0.29 c | 5.18 ± 1.40 b | 1.82 ± 0.08 c | 2.08 ± 0.29 c | 4.28 ± 0.38 cb | 10.52 ± 0.65 a |
| Root | 1.40 ± 0.20 b | 6.47 ± 0.71 a | 2.65 ± 0.29 b | 2.45 ± 0.29 b | 5.42 ± 0.38 a | 6.02 ± 0.23 a |
| Total | 3.36 ± 0.48 c | 11.64 ± 1.73 b | 4.48 ± 0.32 c | 4.53 ± 0.57 c | 9.69 ± 0.69 b | 16.54 ± 0.8 a |
| P (mg) | ||||||
| Shoot | 0.48 ± 0.07 b | 1.68 ± 0.34 a | 0.35 ± 0.02 b | 0.56 ± 0.08 b | 1.69 ± 0.15 a | 1.54 ± 0.09 a |
| Root | 0.37 ± 0.05 b | 1.49 ± 0.16 a | 0.53 ± 0.06 b | 0.88 ± 0.10 b | 1.62 ± 0.11 a | 1.70 ± 0.07 a |
| Total | 0.85 ± 0.12 b | 3.17 ± 0.50 a | 0.88 ± 0.06 b | 1.44 ± 0.18 b | 3.31 ± 0.24 a | 3.24 ± 0.15 a |
| K (mg) | ||||||
| Shoot | 0.37 ± 0.05 c | 3.08 ± 0.62 a | 0.67 ± 0.03 c | 1.48 ± 0.21 bc | 3.10 ± 0.28 a | 2.30 ± 0.14 ab |
| Root | 0.23 ± 0.03 c | 1.41 ± 0.16 a | 0.82 ± 0.09 b | 0.90 ± 0.11 b | 1.21 ± 0.09 a | 1.60 ± 0.06 a |
| Total | 0.60 ± 0.09 c | 4.49 ± 0.77 a | 1.49 ± 0.10 c | 2.38 ± 0.31 bc | 4.31± 0.34 a | 3.89 ± 0.19 ba |
*Bacterial inoculation was done 100 days after sowing of P. montezumae. Hm = H. mesophaeum, C = Cohnella sp., Ab = Azospirillum brasilense. Values with the same letter in the same row are equal according to the Tukey test (P = 0.05). ± Standard error of the mean (n = 4).
The importance of N, P and K acquisition in mycorrhizal plants has been demonstrated and studied (Smith & Read, 2008). For example, recently: i) Kayama, Qu, and Koike (2015) indicated that the presence of ectomycorrhizal fungi stimulated increased N in Larix kaempferi Lamb. plants; ii) Zong et al. (2015) recorded a significantly higher P increase in Pinus densiflora Siebold and Zucc. and Quercus variabilis Blume. inoculated with Pisolithus sp., Cenococcum geophilum Fr. and L. laccata than in uninoculated plants; and iii) Martínez-Reyes et al. (2012) reported a positive effect on K translocation in P. greggii plants inoculated with H. mesophaeum.
In the present study, the fungus Hm and the bacterium Ab showed a synergistic effect reflected in shoot and total N content. Xiao-Qin et al. (2012) also reported that ectomycorrhizal colonization, growth and mobilization of N, P and K increased in P. thunbergii Parl. inoculated with the ectomycorrhizal fungus Boletus edulis Rostk. and the bacterium Bacillus cereus Franklan bacteria, compared to uninoculated plants.
Conclusions
Inoculation of Hebeloma mesophaeum, alone or in combination with Cohnella sp. (C) and Azospirillum brasilense (Ab), improved the growth and physiological quality of Pinus montezumae plants, as the biomass, photosynthetic rate, chlorophyll and carotene concentration, and N, P and K content increased. By contrast, inoculation of C and Ab separately did not show significant effects on the variables evaluated, except for the carotene concentration. A synergistic effect in terms of root biomass and N content was observed in the shoot when inoculated with Hm + Ab. These effects were associated with ectomycorrhizal colonization of 69 to 76 % in treatments inoculated with Hm alone and coinoculated. The present study demonstrates that inoculation with Hm, alone or in combination with the mycorrhizal helper bacteria C and Ab, has potential for use in the production of quality P. montezumae plants, one of the species most used in reforestation and soil restoration programs in Mexico. In addition, Hm inoculation offers the opportunity to harvest edible basidiomas, which constitute a non-timber forest product of economic, social and cultural importance.











 texto en
texto en 


