Introduction
A range site is a unit of land with homogeneous physical and biotic characteristics which respond similarly to a given use pattern. Frequently, different states or range conditions occur within a site, either spatially or temporally, which according Heady and Child (1994) are the current states of vegetation in relation to the site’s potential condition, that is to say, the mature and relatively stable state vegetation in that site. The most important resource in a range is its plant cover; hence, determining its condition provides insights on how it has been managed and the actions to be taken for an optimum use (Heady & Child, 1994; Holechek, Pieper, & Herbel, 2011; Quirk, 2002).
Also, these successive condition changes result in concomitant alterations in the soil/water/vegetation systems (Thurow, 2000). Dry land ecosystems display unique features arising from aridity; dry land communities are less tolerant to the stress imposed by the diverse uses, and take longer to recover. Dry lands deterioration results mainly from the users of dry land resources, who increase their demands on these resources and ultimately degrade them while attempting to improve their living conditions (Griffin, 2002).
All these problems might be happening in relation to the gathering of the pupae state (escamoles) of the ant Liometopum apiculatum Mayr in San Luis Potosí state highlands, where primary industry activities are dominant. This degradation may occur particularly in rangelands under common use tenure (“ejidos”) in the municipality of Charcas, where this activity has expanded rapidly over the past decade because escamoles are highly demanded as a delicacy food, eaten since prehispanic times by many ethnic groups, and currently also in urban markets and restaurants (Ramos-Elorduy, 2006). Further details on this ant species biology and utilization were previously published by Lara-Juárez, Aguirre-Rivera, Castillo-Lara, and Reyes-Agüero (2015). However, as local collectors lack the traditional knowledge for collecting escamoles as in other Mexican states such as Hidalgo and Tlaxcala, and they disregard the relationship between this insect and its surroundings, in terms of feeding, resting, sheltering from the sun or reproducing and building ant nests, gathering seems excessive and careless, which, coupled with overgrazing, may be causing a rapid deterioration of this resource (Lara-Juárez et al., 2015).
Thus, the aim of this work was to relate the structure of the range, through the effect of site and condition, with L. apiculatum ant nest density in an escamoles-collecting area. To this end, the vegetation structure, the state of the soil surface and the number of associated active ant nests were investigated in an area categorized according to the history of use (ejido communal ranges and private ranch), and considering three different physiognomic variants of vegetation (probable sites) that spread over both types of land tenure.
Materials and methods
Study area
The study area comprised the zone where escamoles are collected, which includes ejidos and ranches located in the northeastern portion of the municipality of Charcas, in northern San Luis Potosí state (29.5 km from Charcas city to the study area, Figure 1), where this economic activity started recently and is now actively carried out. This area is characterized by heterogeneous surface lithology, which in turn gives rise to scrub ranges dominated by species associated with igneous or sedimentary substrates. The weighted average temperature and precipitation recorded at the nearest weather stations (Laguna seca, 38 years and Charcas, 54 years) are summarized in Figure 2 (Instituto Nacional de Estadística, Geografía e Informática [INEGI], 2002).

Figure 2: Temperature (---) and precipitation (—) distribution in the study area region (taken from García, 2004). (a) Laguna Seca (2,030 m, 16.8 ºC, 439.2 mm), (b) Charcas (2,021m, 17.6 ºC, 446.5 mm).
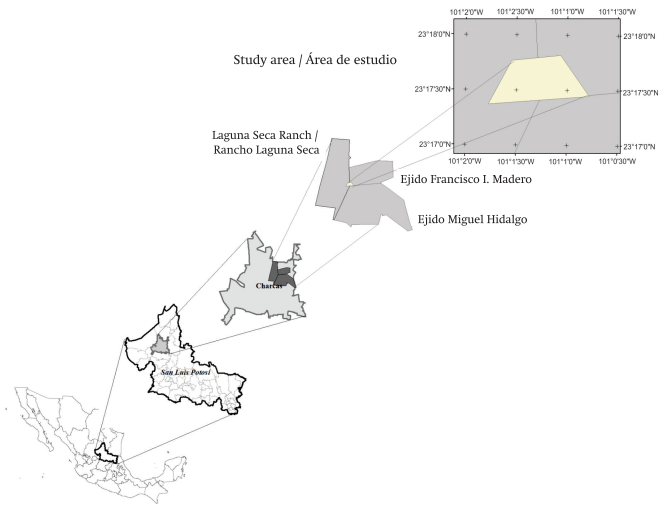
Following the approach of Bolaños and Aguirre (2000), reconnaissance trips were conducted through the area, and based on thematic maps and orthophotos, an area of relatively homogeneous geomorphology (landscape) was located which included both ranches and ejidos (Figure 3); this made it possible to have two contiguous areas, with contrasting range condition and history of use (Figure 4). Then, certain vegetation physiognomy and edaphic differences were visually identified; these differences were sufficient to assume that these corresponded to three range sites, which also showed noticeable differences in terms of ant nest density, to be considered as favorable, fair and unfavorable sites for nest establishment. Thus, the sampling area included a portion of Cerro Blanco pasture in the Francisco I. Madero ejido, as well as part of the Siete Vueltas pasture belonging to the Laguna Seca ranch.
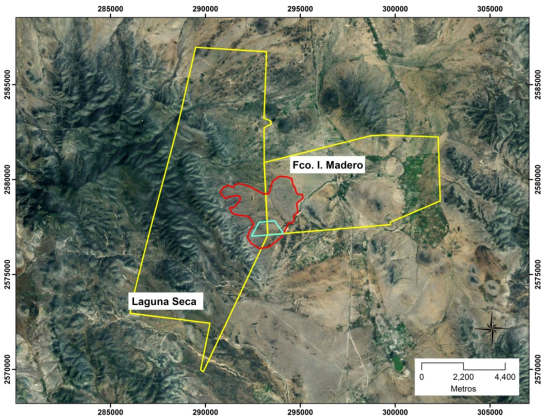
Figure 3: Study area location, Laguna Seca ranch and Francisco I. Madero ejido common land, Charcas, San Luis Potosí (in yellow, property boundary; in red, landscape boundary; in blue, sampling area).
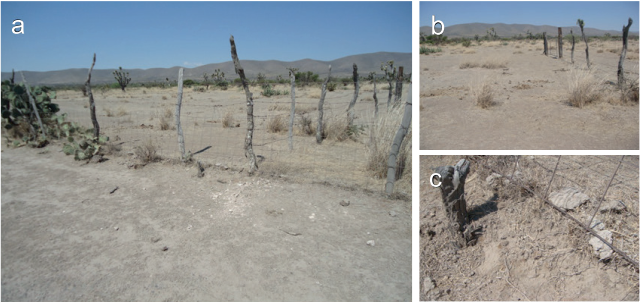
Figure 4: Boundary between the Cerro Blanco fraction of the Francisco I. Madero ejido (left) and the Siete Vueltas pasture of the Laguna Seca ranch (right). (a) The loss of soil in the common land has formed a step over the line of the fence. (b) At the common land lower vegetation density and greater amount of manure can be observed. (c) Approach to the difference in ground level, about 10-12 cm, generated by overgrazing.
Vegetation sampling procedure
Field surveys were conducted between October and December 2011, during a drought of over two years. In order to estimate the structural attributes of vegetation and the density of ant nests, the point-centered quarter method was used along transects, with adjustment for multi-layer shrubby vegetation (Brower, Zar, & von Ende, 1998).
Transects were defined over the images for each of the three range sites, perpendicular to the fence that separates the ranch and the ejido, and spaced at least 100-m from each other to avoid overlapping. As the sites areas were different the number and length of transects were different for each site but identical for each condition. Sampling points number was the same in both conditions and they were distributed along transects and geo located at 100-m intervals to avoid sampling the same ant nest twice from adjacent sites, since the radius of ant activity around the nest was observed to be near 45 to 50-m; sampling points were at least 50-m away from fences (Figure 5). Before starting field measurements, the plant species present were listed and categorized into four strata according to their life forms: herbaceous, lower shrub, upper shrubby and arborescent; then, at each sampling point we measured in sequence the species measurements belonging to each stratum. Also, herbarium specimens were collected to corroborate and support field identifications; after that, they were deposited at the Isidro Palacios herbarium in the Instituto de Investigacion de Zonas Deserticas of the Universidad Autonoma de San Luis Potosi, Mexico. Following the approach of Vallentine (1990) and Bolaños and Aguirre (2000), the plant species were recorded and classified as desirable, less desirable and undesirable, according to their value as forage and successional reaction to grazing.
Plant attributes and ant nests
In a sequence per stratum, in each of the four quadrants of each of the 52 points sampled, we recorded the distance from the point to the nearest ant nest, as well as the closest plant specimen, for which plant height, major and minor diameters of its basal area and cover were recorded; the truncated cone volume formula was used to indirectly estimate the volumetric biomass from these data:
V = 1/3 • π • h (R2 + r2 + R • r)
Finally, the visually appreciated degree of defoliation was recorded. Data for a total of 832 plant specimens and 208 ant nest search quadrants were recorded.
Ground cover
The state or condition of the ground cover was measured with the line intercept method (Brower et al., 1998) to estimate the proportion of the total area occupied by basal plant cover (living or dead), stones, manure, bare soil and ant trails. To this end, five lines (5 m long each) were laid at each range site and condition (three sites, two conditions = 30 in total) using a rope tightened between two stakes; lines were fixed in an alternating and parallel manner to the sampling transects, on both sides and 10-m away from them.
Data analysis
Absolute and relative density and volumetric biomass per hectare were calculated for each plant species, as well as ant nest density and the proportion of each cover category identified on the ground cover. To support the respective tabular analyses, structural values were averaged for the total species recorded; to compare sites and conditions only data for plant species with more than ten numerical records were used. A completely randomized design with a 2×3 factorial arrangement of treatments was used: Factor A was the range condition (private ranch and ejido). Factor B was the range site (favorable, fair and unfavorable). Figures for bare soil, mulch, vegetation, stones and manure, expressed in percentages, were transformed to arcsin and analyzed with the GLM procedure of SAS (Statistical Analysis System, 1999), with a significance level of P < 0.05; Tukey’s test was used to compare statistically significant mean values.
Results and discussion
Table 1 shows the plant species recorded and evaluated in the municipality of Charcas, San Luis Potosí. Using the same sample size and sampling design, the ejido recorded only 22 plant species versus 35 plant species recorded in the ranch. The ejido’s overall floristic composition was also recorded at the ranch, but lacked several shrubby and herbaceous forage species which are still present in the ranch. As a result of the contrasting grazing history, the overall plant species density in the ejido was only 42.8 % of that in the ranch; by contrast, volumetric biomass in the ranch was only 27.92 % of the one recorded in the ejido. This is explained by the predominance of bigger and long-lived species unsuitable for grazing in the ejido’s heavily deteriorated range sites; this finding agrees with reports from Veracruz, Mexico by Campbell, Jarillo-Rodríguez, López-Ortiz, and Castillo-Gallegos (2013), who mention that the increase of cattle in a pasture leads to an increasing abundance of plant species with poor forage quality, while perennial grasses tend to decrease. Otherwise, abundant -although smaller- herbaceous species were recorded at the ranch, such as grasses (Tables 2 and 3). Finally, only two active ant nests were recorded in the ejido (in the favorable site), in contrast with 14 found in the ranch. In this regard, Rojas and Fragoso (2000) point out that the absence of ant communities in the central Chihuahuan Desert is an indicator of deterioration. Similarly, Hoffmann (2000) mentions that the richness of ant species is negatively correlated with grazing intensity; in contrast, studies made in intensive silvopastoral systems in Colombia by Rivera, Armbrecht, and Calle (2013) found that richness of ant species can be increased with the presence of woody vegetation such as Leucaena leucocephala (Lam.) De Wit. Nests of some ants (harvester ants: Messor and Pogonomyrmex) are well documented as representing nutrient-rich patches and as supporting higher biomass and species richness of annual plants on the periphery of the nest discs (Whitford, Barness, & Steinberger, 2008). Bestelmeyer and Wiens (1996) found in an extended gradient ground foraging from highly degraded range condition to a less severe disturbance by traditional grazing practices in the Chaco of northern Argentina, changes in the ground-layer vegetation from sparse to a structurally complex mixture of grasses and forbs, as well as higher ant species richness in the better range condition.
Table 1: Species recorded through the vegetation sampling in the study area.
| Species | Life forms | |||
|---|---|---|---|---|
| Herbaceous | Lower shrub | Upper shrub | Arborescent | |
| Acacia schaffneri (S. Watson) F.J. Herm. | x | |||
| Agave salmiana Otto ex Salm-Dick | x | |||
| Aristida adscensionis L. | x | |||
| Berberis trifoliolata Moric. | x | |||
| Bouteloua gracilis (Kunth) Lag. ex Griffiths | x | |||
| Bouteloua reederorun Columbus | x | |||
| Bouvardia ternifolia (Cav.) Schldl. | x | |||
| Calliandra eriophylla Benth. | x | |||
| Condalia Cav. | x | |||
| Cylindropuntia imbricata (Haw.) F. Knuth | x | |||
| Dalea bicolor Humb. & Bonlp. ex Willd. | x | |||
| Dasyochloa pulchella (Kunth) Willd. ex Rydb. | x | |||
| Echinocactus platyacanthus Link & Otto | x | |||
| Euphorbia heterophylla L. | x | |||
| Ferocactus pilosus (Salm-Dick) Werderm | x | |||
| Hilaria cenchroides Kunth | x | |||
| Ipomoea orizabensis (Pelletan) Ledeb. ex Steud. | x | |||
| Jatropha dioica Sessé ex Cerv. | x | |||
| Larrea tridentata (Sessé & Moc. ex DC.) Cov. | x | |||
| Leptochloa dubia (Kunth) Nees | x | |||
| Menodora coulteri A. Gray | x | |||
| Mimosa biuncifera Benth. | x | |||
| Muhlenbergia villosa Swallen | x | |||
| Opuntia cantabrigiensis Lynch | x | |||
| Opuntia leucotricha DC. | x | |||
| Opuntia rastrera F. Weber | x | |||
| Opuntia streptacantha Lem. | x | |||
| Parthenium incanum Kunth. | x | |||
| Prosopis laevigata (Willd.) M. Johnston | x | |||
| Rhus microphylla Engelm. | x | |||
| Salvia ballotiflora Benth. | x | |||
| Senna bauhinioides (A. Gray) H.S. Irwin & Barneby | x | |||
| Solanum ehrenbergii (Bitter) Rydb. | x | |||
| Sporobolus airoides (Torrey) Torrey | x | |||
| Yucca L. | X | |||
Table 2: Vegetation structure in soil sites (favorable, fair, unfavorable) in the Laguna Seca ranch, considered with better rangeland condition for the establishment of escamoles (Liometopum apiculatum) ant nests.
Table 3: Vegetation structure in soil sites (favorable, fair, unfavorable) of ejido Francisco I. Madero, considered with better rangeland condition for the establishment of escamoles (Liometopum apiculatum) ant nests.
| Botanical composition | Biomass (m3·ha-1) | Total | Density (individuals·ha-1) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Favorable | Fair | Unfavorable | Favorable | Fair | Unfavorable | |||
| Acacia schaffneri | 499.8 | 5,907.0 | 3,200.8 | 9,607.6 | 2,606.4 | 3,418.7 | 4,710.0 | 10,735.1 |
| Agave salmiana | 0.0 | 0.0 | 23.7 | 23.7 | 0.0 | 0.0 | 210.6 | 210.6 |
| Echinocactus platyacanthus | 0.0 | 9E-04 | 0.0 | 9E-04 | 0.0 | 6.5 | 0.0 | 6.5 |
| Bouteloua gracilis | 4E-04 | 9.8E-05 | 2.5E-03 | 3E-03 | 13.1 | 7.9 | 10.4 | 31.4 |
| Bouteloua reederorun | 0.0 | 0.0 | 2E-03 | 2E-03 | 0.0 | 0.0 | 35.4 | 35.4 |
| Cylindropuntia imbricata | 50.0 | 101.0 | 141.3 | 292.3 | 0.1 | 0.5 | 1.1 | 1.70 |
| Dalea bicolor | 0.0 | 0.0 | 12E-02 | 12E-02 | 0.0 | 0.0 | 5.6 | 5.6 |
| Euphorbia heterophylla | 0.0 | 0.0 | 4.4E-03 | 4.4E-03 | 0.0 | 0.0 | 26.0 | 26.0 |
| Hilaria cenchroides | 0.0 | 1E-04 | 0.0 | 1E-04 | 0.0 | 6.7 | 0.0 | 6.7 |
| Jatropha dioica | 26E-02 | 0.0 | 1.3 | 1.6 | 363.9 | 0.0 | 403.0 | 766.9 |
| Larrea tridentata | 158.2 | 207.6 | 289.6 | 655.4 | 1,512.7 | 1,452.3 | 5,366.6 | 8,331.6 |
| Menodora coulteri | 1.3E-03 | 3.8E-03 | 0.0 | 5.1E-03 | 94.7 | 115.2 | 0.0 | 209.9 |
| Opuntia leucotricha | 0.0 | 0.0 | 3.9E-03 | 3.9E-03 | 0.0 | 0.0 | 12.9 | 12.9 |
| Opuntia rastrera | 3.0 | 5.0 | 5.5 | 13.5 | 9.4 | 46.4 | 208.4 | 264.2 |
| Opuntia streptacantha | 0.0 | 0.0 | 279.8 | 279.8 | 0.0 | 0.0 | 771.9 | 771.9 |
| Parthenium incanum | 11E-02 | 7E-02 | 0.0 | 0.18 | 20.1 | 12.7 | 0.0 | 32.8 |
| Parthenium sp. | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 19.1 | 0.0 | 19.1 |
| Prosopis laevigata | 0.0 | 31.9 | 9,294.3 | 9,326.2 | 0.0 | 450.0 | 1,213.1 | 1,663.1 |
| Senna bauhinioides | 4E-04 | 1.9E-03 | 2.7E-03 | 5E-03 | 17.0 | 36.9 | 114.9 | 168.8 |
| Sporobolus airoides | 0.0 | 0.1 | 1.0 | 1.1 | 0.0 | 153.0 | 262.5 | 415.5 |
| Dasyochloa pulchella | 1E-03 | 0.0 | 2E-03 | 3E-03 | 428.3 | 0.0 | 1,927.1 | 2,355.4 |
| Yucca sp. | 872.4 | 1,230.6 | 25,744.4 | 27,847.3 | 2,530.6 | 2,257.4 | 949.4 | 5,737.4 |
| Total | 1,583.8 | 7,483.4 | 38,982.0 | 48,049.2 | 7,596.4 | 7,983.3 | 16,228.9 | 31,808.6 |
The prolonged overgrazing in the ejido has resulted in profound changes in vegetation structure, reducing the original differences between range sites in it. Therefore, for the structural contrast between range sites recognized by their physiognomic differences and apparent ant nest abundance, only the data recorded at the ranch seem valid (Table 2). Thus, the results of the overall survey-without removing those species with less than 10 entries, with average figures likely skewed, especially regarding density- reveal various differences in plant species composition, volumetric biomass and plant density of species common to two or three range sites. These differences between range sites are also observed in cumulative structural figures, particularly in the case of density, and notably in ant nest abundance: 12 in the favorable site (six•ha-1), two in the fair site and none in the unfavorable site (Figure 6).
The species likely related positively to the escamoles ant were Bouteloua gracilis (Kunth) Lag. ex Griffiths, Menodora coulteri A. Gray, Hilaria cenchroides Kunth, Leptochloa dubia (Kunth) Nees, Muhlenbergia villosa Swallen and Parthenium incanum Kunth (Table 2), all displaying a decreasing response to grazing and, therefore, regarded as desirable. In sites located in the Chihuahua desert, Bestelmeyer and Wiens (2001) also found higher abundance of several ant species in ranges dominated by B. gracilis than in sites dominated by other plant species such as B. eriopoda (Torr.) Torr., Prosopis glandulosa Torrey and Yucca elata Engelm. The worse range condition (ejido) evidenced a high abundance of the undesirable creosote bush (Larrea tridentata [Sessé & Moc. ex DC.] Cov.), which is consistent with findings reported by Whitford and Steinberger (2011) on the invasion and expansion of L. tridentata in heavily overgrazed ranges of the southern United States and northern Mexico. To get a more robust estimate of the vegetation structure and dynamics, plant species with less than 10 records were removed from the analysis; the remaining species were categorized into desirable, less desirable and undesirable. With the notable exception of Aristida adscensionis L., a species usually classified as less desirable and present only in the private land, the species composition of the most abundant species (i. e. those with ≥ 10 records) was identical in both areas with a contrasting range condition (Tables 4 and 5). In contrast, the respective density values (Table 4) showed different patterns as a reaction to the very different stocking rate, livestock types and selectivity. Thus, four of the six species classified as desirable displayed a markedly decreasing density (M. coulteri, B. gracilis, B. reederorun Columbus and Dalea bicolor Humb. & Bonlp. ex Willd.); the other two species of this group were relatively unaffected by deterioration, similarly to the undesirable species Jatropha dioica Sessé ex Cerv. and Cylindropuntia imbricata (Haw.) F. Knuth. In contrast, the rest of the less desirable and undesirable species displayed densities several times higher in the ejido land with the worse range condition, that is, their abundance has clearly risen in response to overgrazing. Indeed, Milton and Dean (2010) and Whitford and Steinberger (2011) point out that overgrazing makes pastures more vulnerable to invasion by undesirable species, such as L. tridentata, C. imbricata and Prosopis spp.
Finally, it is worth noting that under the better range condition (ranch), the total density of both desirable and less desirable species was several times higher than under the worse range condition (ejido); the opposite occurred in the case of undesirable species (Tables 4 and 5). Similarly, Del Curto, Porath, Parsons, and Morrison (2005) mention that private ranges host a higher abundance of desirable plants and display less severe effects of overgrazing relative to public ranges, mainly due to better management practices such as a more even distribution of livestock and watering places, seasonal grazing and grazing duration, and the use of supplements and electrified fences. Ramírez and Enríquez (2003) remark that the richness, diversity and ant community composition in two tropical silvopastoral systems composed mainly of Cynodon plectostachyus (K. Schum.) Pilg., associated with Prosopis juliflora (Sw.) DC. (2-3 heads•ha-1) or with L. leucoephala (4-4.5 heads•ha-1), are negatively affected by the stocking rate.
Table 4: Effect of range condition on the density of species with more than 10 records in the sampling of Charcas, San Luis Potosí.
| Forage value | Species | Better condition (ranch) | Worse condition (ejido) | ||
|---|---|---|---|---|---|
| Absolute (individuals·ha-1) |
Relative (%) | Absolute (individuals·ha-1) |
Relative (%) | ||
| Desirables | Sporobolus airoides | 430.1 | 0.78 | 415.5 | 2.55 |
| Parthenium incanum | 18.3 | 0.03 | 32.8 | 0.20 | |
| Menodora coulteri | 1,863.4 | 3.38 | 209.9 | 1.29 | |
| Bouteloua gracilis | 7,389.0 | 13.44 | 31.4 | 0.19 | |
| Bouteloua reederorun | 1,761.1 | 3.20 | 35.4 | 0.21 | |
| Dalea bicolor | 223.1 | 0.40 | 5.6 | 0.03 | |
| Subtotal | 11,685.0 | 21.25 | 730.8 | 4.49 | |
| Less desirables | Dasyochloa pulchella | 1,538.5 | 2.79 | 6,214.0 | 38.22 |
| Agave salmiana | 37.2 | 0.06 | 210.6 | 1.29 | |
| Aristida adscensionis | 40,271.3 | 73.26 | - | - | |
| Subtotal | 41,847.1 | 76.12 | 6,424.6 | 39.51 | |
| Undesirables | Larrea tridentata | 625.1 | 1.13 | 8,331.5 | 51.25 |
| Jatropha dioica | 807.0 | 1.46 | 769.2 | 4.73 | |
| Cylindropuntia imbricata | 4.9 | 0.009 | 1.7 | 0.010 | |
| Sub total | 1,437.1 | 2.61 | 9,102.6 | 55.98 | |
| Total | 54,969.5 | 100 | 16,258.1 | 100 | |
Table 5 Effect of range condition on the volumetric biomass of species with more than ten records in the sampling of Charcas, San Luis Potosí.
| Forage value | Species | Better condition (ranch) | Worse condition (ejido) | ||
|---|---|---|---|---|---|
| Absolute (individuals·ha-1) |
Relative (%) | Absolute (individuals·ha-1) |
Relative (%) | ||
| Desirables | Sporobolus airoides | 5.8 | 2.36 | 1.1 | 0.22 |
| Parthenium incanum | 1.5 | 0.61 | 0.1 | 3.6E-02 | |
| Menodora coulteri | 5.8E-02 | 0.02 | 5E-03 | 1E-03 | |
| Bouteloua gracilis | 0.012 | 0.04 | 3E-03 | 6E-04 | |
| Bouteloua reederorun | 2.2E-02 | 8E-03 | 2E-03 | 4E-04 | |
| Dalea bicolor | 29.4 | 11.96 | 0.012 | 2.4E-02 | |
| Subtotal | 36.9 | 15.01 | 1.4 | 0.29 | |
| Less desirables | Dasyochloa pulchella | 2E-03 | 1E-03 | 3E-03 | 6E-04 |
| Agave salmiana | 34.5 | 14.01 | 23.7 | 4.87 | |
| Aristida adscensionis | 1.8E-02 | 7E-03 | - | - | |
| Subtotal | 34.5 | 14.06 | 23.7 | 4.88 | |
| Undesirables | Larrea tridentata | 142.6 | 58.03 | 318.9 | 65.54 |
| Jatropha dioica | 1.0 | 0.41 | 0.078 | 0.16 | |
| Cylindropuntia imbricata | 30.5 | 12.44 | 141.6 | 29.10 | |
| Sub total | 174.2 | 70.89 | 461.4 | 94.84 | |
| Total | 245.7 | 100 | 486.5 | 100 | |
As regards volumetric biomass (Table 5), the key finding is that the range with the worse condition (ejido) recorded twice the amount of biomass relative to the range site with the better condition (ranch). Likewise, in the ejido almost 95 % of biomass belonged to undesirable species, whereas the biomass of desirable species was negligible (0.3 %) in this range, and relatively low (15 %) in the ranch. Thus, the deterioration derived from overgrazing leads to the dominance of the more massive and less productive plant species, with predominance of less active biomass (wood) that is unsuitable for grazing (Odum & Sarmiento, 1998). Therefore, this instantaneous structural estimate without successional or dynamic considerations may lead to mistaken assessments on the health or condition of a range site.
As opposed to density, the analysis of biomass revealed that all desirable plant species showed a clear decreasing response to overgrazing, contrasting with the significant increase of C. imbricata, also documented by Milton and Dean (2010) and Zimmermann (2010); in contrast, the biomass of J. dioica was not related to deterioration. Fulbright, Lozano-Cavazos, Ruthven III, and Litt (2013) point out that J. dioica is present in areas with a history of disturbance for over 30 years.
Ground cover
Figure 6 summarizes the results of the effects of range site and range condition on soil surface cover. Regardless of the range site, the ejido displayed as an average more than twice the area of bare soil and manure, 60 % of the living basal cover and less than 30 % of the mulch recorded in the ranch.
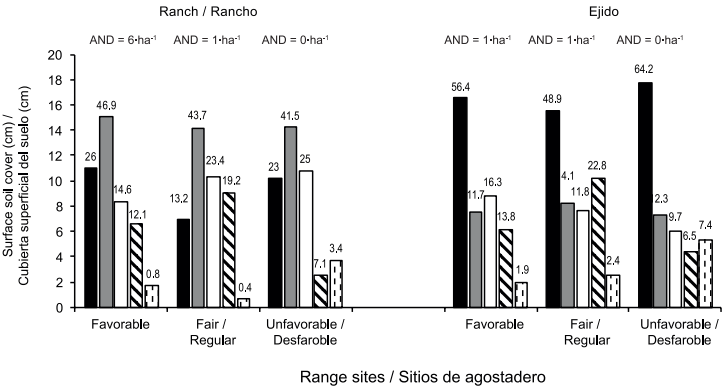
Figure 6 Effect of range condition and tentative site on the surface soil cover of Laguna Seca
ranch and ejido Francisco I. Madero of Charcas, San
Luis Potosí (n = five Canfield lines of 5 m each). Bare soil
( ), mulch
(
), mulch
( ),
vegetation basal cover (
),
vegetation basal cover ( ), rocks
(
), rocks
( ) and feces
(
) and feces
( ). Numbers
at the top of each bar refer to %. AND = Ant nest density. Values in
the bars are represented by the transformed data.
). Numbers
at the top of each bar refer to %. AND = Ant nest density. Values in
the bars are represented by the transformed data.
A two way ANOVA detected a significant difference on the range condition for bare soil variable (F1,32 = 48.05, P < 0.0001); effects of range site and the interactions were not significant (F2,32 = 2.79, P = 0.0763 and F2,32 = 0.47, P = 0.6269, respectively). The lowest bare soil percentages were observed in the three range sites of the private ranch (favorable, fair and unfavorable sites), 26, 13.2 and 23 % respectively.
Mulch was affected by the range condition (F1,32 = 55.46, P < 0.0001); effects of range site and the interaction were not significant (F2,32 = 0.16, P = 0.8470 and F2,32 = 0.20, P = 0.8184, respectively). The lowest mulch percentages were recorded in the three range sites of the ejido (favorable, fair and unfavorable sites), 11.7, 14.1 and 12.3 % respectively.
Vegetation basal cover presented effect of the range condition (F1,32 = 5.18, P < 0.0275); effects of range site and the interaction were not significant (F2,32 = 0.08, P = 0.9253 and F2,32 = 2.11, P = 0.1372, respectively). The lowest vegetation basal cover percentages were observed in the three range sites of the private ranch (favorable, fair and unfavorable sites), 14.6, 23.4, and 25 %, respectively.
A two way ANOVA detected effect of the range site for the stones variable (F2,32 = 4.57, P = 0.0179); no significant differences were found for range condition, and interaction (F1,32 = 0.18, P = 0.6708; and F2,32 = 0.10, P = 0.9077, respectively). The higher values were found in the fair range sites in both range conditions private ranch and ejido, (22.8 and 19.2 %, respectively).
The feces variable presented effect of the range site (F2,32 = 2.56, P < 0.0029); effects of the range condition and the interaction were not significant (F2,32 = 7.06, P = 0.1196 and F2,32 = 0.39, P = 0.6823, respectively). The lowest feces percentages were observed in the favorable and fair range sites of the two conditions (ranch and ejido), 0.8, 0.4, 1.9 and 2.4 % respectively.
Since in both areas livestock have grazed freely and preferentially on certain plant species in the three range sites, given the lack of fences, the current differences in the state of soil surface are partly derived from differing degrees of historical range utilization. However, range site considered favorable for escamoles ants in both areas only consistently differed by recording the lowest manure cover -an indicator of lower cattle disturbance; indeed, the greater the intensity and continuity of trampling, the higher the likelihood of ant-trail destruction and crushed ants.
When the effect of range condition on ground cover was compared, the ejido, with only two ant nests recorded, showed a higher proportion of bare soil (200 %) and manure (224 %), and a lower proportion of mulch (25 %) than the ranch which had a better condition and 14 ant nests; however, the proportions of vegetated area (basal area) and stones were similar in both conditions. These findings agree with those reported by Nash et al. (2001), who mention that the abundance of ant communities decreases as the condition of range sites changes from good to poor, and that this is directly related to the proportion of bare soil and the reduction of plant cover.
Conclusions
In the ranges analyzed, the contrasting range condition that results from the pattern of use was clearly documented. Range sites which were distinctive in terms of physiognomic differences and apparent ant nest density (favorable, fair and unfavorable) show the same overall plant composition and total instantaneous biomass; however, the density and biomass of desirable species and the number of ant nests found indicate that these areas differ from each other in spite of sharing the same history of use. The relationship between the density of Liometopum apiculatum ant nests and both the vegetation type and range condition, and the ground cover, was evidenced by recording the highest ant nest density (14•ha-1) in the favorable site in the ranch (better range condition). Thus, as long as the range has the environmental factors required by L. apiculatum and its condition is good, the density and quality (size and ant activity) of ant nests will tend to their optimum, as the resources and environmental conditions required are available for colonies of this insect to develop normally, even under a rational grazing pressure.











 texto en
texto en