Introduction
Fructans are reserve polysaccharides that some plants synthesize from sucrose and fructose molecules (Banguela & Hernández, 2006). Fructans of vegetable origin have from a lower degree of polymerization (DP, ≤ 200 fructose molecules) than those of microbial origin; and more complex structures (Olvera, Castillo, & López-Munguía, 2007) and differ from one another due to their structure and GP (López, Mancilla-Margalli & Mendoza-Díaz, 2003; Chalmers et al., 2005; Mancilla-Margalli & López, 2006; Waleckx, Gschaedler, Colonna- Ceccaldi & Monsan, 2008).
Fructans have been present in the human diet for thousands of years, through the consumption of vegetable organs where polysaccharides are stored as reserve substances (Leach, 2007; Leach & Sobolik, 2010). Presently, the fructans with greatest importance worldwide are inulin and oligofructoside. The latter is obtained from the partial enzymatic hydrolysis of the inulin extracted from the chicory root (Cichorium intybus L.) (Coussement, 1999). The innocuousness of inulin and oligofructoside for their use in the elaboration of food has been listed within substances denominated "Generally Recognized as Safe" (GRAS) in the USA and as "food ingredients and not additives" in the European Union (Coussement, 1999).
There is a growing demand for commercial fructans as ingredients of processed food for daily consumption (Kays & Nottingham, 2007). In addition, fructans are used as textural and consistency enhancer ingredients for food products, replacing starch and grease, thus reducing the energetic content of the food (Franck, 2002). Fructans pass intact through the digestive tract without any caloric intake in the blood (Niness, 1999); in addition, they have prebiotic properties, as they constitute the specific substrate for Lactobacillus and Bifidobacterium bacteria genus of the colon, which produce changes that benefit the health of the host (Gibson & Roberfroid, 1995). Thus, the consumption of fructans improves the performance of the digestive tract, prevents constipation, helps with the absorption of some minerals such as calcium and reduces the risk of cardiovascular diseases, diabetes and colon cancer (Greg, 2009).
In Mexico, inulin was first imported in 2000 (13.3 t) (Instituto Nacional de Estadística y Geografía [INEGI], 2001), and in 2011 3,338.24 t were already registered (INEGI, 2012). At the same time, in 2001 24.5 t of inulin and in 2011 1,123.07 t were exported. Imports and exports remain registered as inulin, but it is deduced that the imported inulin comes from chicory and the exportations are maguey fructans, mainly from Agave angustifolia Haw. spp. tequilana Valenzuela-Zapata & Nabhan, incorrectly denominated "Agave inulin".
The first evidence of the presence of fructose polysaccharides in the Agave species was provided by Sánchez-Marroquín and Hope (1953). Then, López et al. (2003) established the molecular structure of the fructans of maguey for tequila and found the presence of branched structures and a higher level of polymerization than those of chicory. This triggered a series of works about the properties, extraction processes, variation and biological evaluation of the reserve compounds of the Agave species, particularly of the maguey for tequila (López, Huazano-García, García-Pérez, & García-Vieyra, 2014). In the maguey plants, the chronological age or the size are less useful than the physiological ripeness in order to recognize their maximum accumulation of reserve substances (fructans), and at the same time, their lower content of saponins (Aguirre, Charcas, & Flores, 2001). The start of the flowering stalk (quiote) marks the end of the formation of leaves and the beginning of the depolymerization of the fructans, or, the consumption of the reserve substances in the formation and sustenance of the reproductive organs (Aguirre et al., 2001). It is fundamental to acknowledge the vegetative to reproductive change, known as quiotillo stage in the highlands of San Luis Potosí, for the rational use of the maguey (Aguirre et al., 2001). Another very important factor for the quality of the fructans is if the entire head of the maguey is used (comprised by the stem and the bases of the leaves) as raw material, instead of only the stem, the organ of the plant where the reserve substances are accumulated (Michel-Cuello, Juárez-Flores, Aguirre-Rivera, & Pinos-Rodríguez, 2008). When the entire head is processed as has been the appropriate procedure for other purposes, it requires more complex and expensive operations to eliminate other existing compounds at the base of the leaves.
According to Mora-López, Reyes-Agüero, Flores-Flores, Peña-Valdivia, and Aguirre-Rivera (2011), Agave salmiana Otto ex Salm-Dyck is the maguey specie with the broadest distributions in the Mexican highlands. More specifically in the semi-arid regions of the highlands of San Luis Potosí, this maguey is an abundant underused spontaneous resource, a natural attribute that increase its value (Aguirre et al., 2001) and can be easily added to products, such as fructans. Some recent works about A. salmiana have been focused on enzymatic hydrolysis (Michel-Cuello et al., 2012), cleansing of monosaccharides (Moreno, 2013), drying of the fructans extracts, as well as functional evaluation in vitro (Moreno-Vilet et al., 2014) and in an animal model (Dávila-Céspedes et al., 2014). However, fructans extraction has yet to be simplified and standardized, as well as the characterization of the final product. Based on the foregoing, the objective of this work was to evaluate the yield and the quality of the fructans concentrate from ripe stems of A. salmiana, extracted in the simplest and most natural manner.
Materials and methods
The extraction process was done based on the high water solubility of the fructans, which allows the collection of a natural extract as it does not require chemical processes and reactions to be separated. Temperatures lower than 55 °C were applied during the entire process to avoid thermal hydrolysis.
Biological material
In the paddock used for the collection of maguey at the Miguel Hidalgo Ejido (Charcas, San Luis Potosí), to supply to the Laguna Seca mezcal factory, five maguey heads were selected, all different sizes but with physiological ripeness features. The heads were taken to the factory yards and were weighed (Básculas Nuevo León D13, Mexico); each one was divided into stem and leaf bases and the parts were weighed separately. Subsequently in the laboratory, each stem was divided into three similar parts to weigh (Tecnocor PPN-30, Mexico) and process them individually on the same day of their collection. At the same time, a sample of the stalk bases was taken (10 % of the weight of the head) to determine the content of dry matter, with the objective of referring the yield of the fructan concentrate based on the fresh and dry weight of the head of the maguey, the present form of use.
Fructans concentrate (FC) extraction
One third of the stem was used to estimate the content of dry matter. The second third was subjected to a juice extractor (International EX-S, Mexico) to separate the liquid extract considered the primary juice and the solid residue or bagasse. The juice was filtrated with two muslin filters, the pH was measured with a potentiometer (Hanna Instruments H198127, Italy) and the soluble solids (°Bx) with a digital refractometer (Reichter AR200, USA). The primary juice was stabilized with the same amount of distilled water in a water bath at 53 ± 2 °C for 60 min as the aqueous extract decomposes quickly, even after one day in refrigeration, as it also happens with the raw extract of chicory (Van Arkel et al., 2014). Immediately, the primary juice was placed in airtight containers and was frozen at -15 °C (Torrey CHTC25, USA). The bagasse of the primary juice was squeezed in a stainless steel gadget designed for the extraction of juices at a constant pressure of 14 kg·cm-2 through a 30 t hydraulic press (Urrea 2471, Spain). The juice recovered from the bagasse was designated as secondary juice, and much like the primary juice, it was filtered and its pH and °Bx were measured; it was stabilized and then frozen. Subsequently, the pressed bagasse was rehydrated with a volume of distilled water equal to the obtained juice (primary juice + secondary juice) and it was stabilized in a water bath; then it was pressed and the generated extract was processed as the previous two, designating this the tertiary juice. It was previously established that the rehydrated and squeezed bagasse more than twice contributed very little (2 %) to the total extract. Due to the lack of equipment for vacuum drying of large samples, a partial evaporation of the primary, secondary and tertiary juices, separately, was done in a cooking and vacuum impregnation equipment (J. P. Selecta Gastrovac, Barcelona, Spain) for the desiccation of the extracts. The original characteristics of the equipment prevented the complete dehydration of the extracts; it was therefore necessary to change the original cap of methacrylate for a flat, stainless steel cap with a vapor exit on the center and a sealed shirt, to introduce a thermometer in the vaporization area. Furthermore, an external condenser was adapted between the exit of vapors and the vacuum line. All juices were partially evaporated, independently, at 75 % of the total vacuum and 42 ± 1 °C, until obtaining 20 % of the original volume or until the extract had a dense consistency of approximately 50 °Bx. The resulting condensation rate was 100 mL of water per 10 min. Subsequently, the concentrated juices were poured in silicone molds (for baking) with a minimum thickness for drying (approximately 95 % DM) in a forced air oven (Shel-Lab FX-14, USA) at 53 ± 2 °C, which lasted around 35 h. The dry extract was removed from the molds and remained in scale shapes, which were grinded in a mortar, sieved, weighed and stored in air tight containers.
The remaining third of the stem was subjected to extraction just as the last one, but the thawed juices were centrifuged at 4,000 rpm (Damon PR-6000, USA) to be decanted and dry the supernatant and the sediment separately, as it was observed that the thawed juices left a white sediment at the bottom of the containers which could facilitate the cleansing of the extract. In order to evaluate the quality of the fructans, an homogenous mix was done with the concentrates obtained from the primary, secondary and tertiary juices of the second third of the stem, a sample was taken and it was fully dried in a forced air oven at 35 ± 2 °C at a constant weight. The humidity free sample was placed in containers and stored in a desiccator until further analysis. The aforementioned procedure was also followed for the samples of concentrates of the supernatant and sediment derived from the last third of the stem.
Evaluation of the relative and absolute fructan yield
The relative and absolute fructan yield was calculated based on the recorded weights (Ohaus 5120, USA) of the fresh and dry matter, both from the stem and the entire head.
Stem humidity content
A sample of 100 g of the third of each stem was weighed (Ohaus 5120, USA) and dried at a constant weight in a forced air oven at 80 ± 2 °C (Shel-Lab FX-14, EE. UU.), to estimate the humidity content. Each measurement was done in triplicate.
Total ash content of the fructan extract
The total ashes were obtained through the burning of the samples. For this, 5 g of the humidity free fructans were weighed (Sartorius BP221S, Germany), which were also calcined in a muffle (Lindberg 51844, Mexico) at 550 °C for 2 h. The sample was cooled and placed in a desiccator and weighed in an analytical balance (Sartorius BP221S, Germany). The percentage of total ashes was obtained through the difference of weights in regards to the initial weight. Each measurement was done in triplicate.
Quantification of sapogenins of the fructan concentrate
The extraction of sapogenins was based on the methodology described by Zamora et al. (2010). For this, 30 mg of sample were subjected to acid hydrolysis and were gauged in a 10 mL volumetric flask. Total of 2 mL of that solution and 2 mL of H2SO4 (2 N) were put in a test tube. The mix was heated at 110 °C for 1.5 h in a dry bath (Labnet International Inc. D1200, USA). After the tubes reached room temperature, an extraction with 5 mL of ethyl acetate was done. The tubes were agitated in a vortex (Barnstead Termolyne M37615, Iowa, USA), submerged in an ultrasonic bath (Bransonic 1510R- MT, Danbury, USA) for 1 min, and the organic cape was separated in another test tube. The sapogenins were quantified according to the method described by Baccou, Lambert, and Sauvaire (1977), using a standard curve 2.5, 5, 10, 15 and 20 µg·mL-1 of diosgenin (Sigma-Aldrich, USA). The absorption readings were done at a wave longitude of 430 nm in a spectrophotometer (HP- Agilent Technologies 8453, Germany).
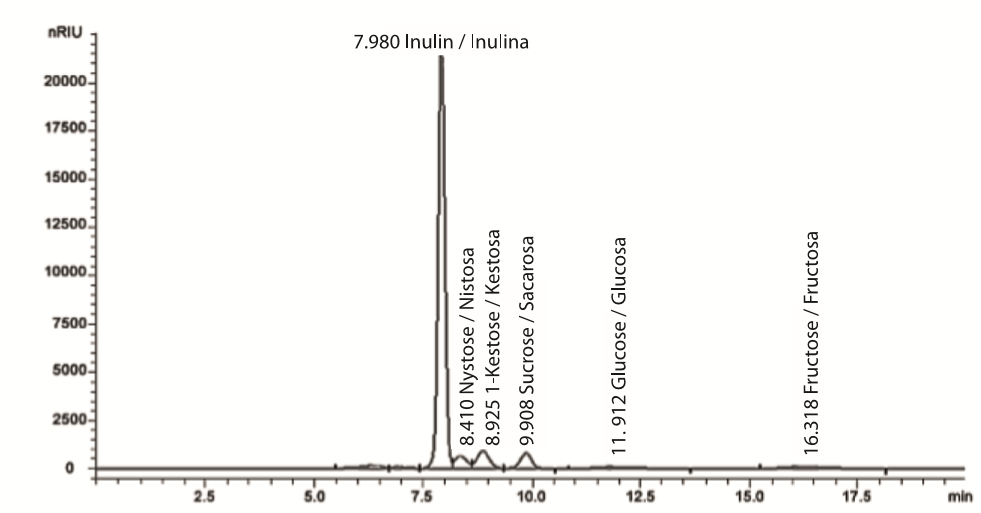
Quantification of carbohydrates through high performance liquid chromatography (HPLC)
The carbohydrates present in the extracts were quantified according to the method described by Zuleta and Sabucetti (2001). Unlike the high performance anion exchange chromatography with amperometric pulse detector (HPAEC-PAD), the method used does not manage to separate individually the components with more than five degree of polymerization, but it can separate mono, bi, tri, and tetrasaccharides so that fructans with a polymerization level higher than five are integrated into one area. For this case, the standard used as reference was inulin (Sigma-Aldrich, USA). This method has been used for the analysis of fructooligosaccharides and inulin in alimentary products, for being practical and fast (Van Loo, Coussement, De Leenheer, & Hoebregs, 1995). Consequently, a chromatograph (HP-Agilent Technologies 1100 series, Germany) with a deaerator, quaternary bomb, thermal compartment and a refractive index detector was used in reverse. An ion exchange column was used on a stationary phase (HP-Animex 78-C [7.8 mm d. i. x 300 mm] Bio Rad, USA). As part of the mobile phase, HPLC grade water (Fermont, México) was used with a flux of 0.5 mL·min-1. The temperature of the column was 75 °C and that of the detector was 50 °C with an operation time per sample of 20 min. The samples were adjusted to a pH of 7 before their injection. Carbohydrates were identified based on the comparison of the retention times with the corresponding reference standards: inulin, sucrose, glucose, fructose (Sigma-Aldrich, USA), nystose and 1-Kestose (Wako Laboratory Chemicals, Japan), all with a purity higher than 98 % (Figure 1). A lineal calibration curve for each reference standard was constructed for the quantification of carbohydrates (r ≥ 0.994) and its linearity constant (slope) was determined. The quantification limit for fructose and glucose (carbohydrates found in a lower proportion in the samples) was 0.02 mg·mL-1 and 0.07 mg·mL-1, respectively.
Results and discussion
Extraction of fructans
With the extraction method based on simple operations and processes, such as the use of a juice extractor and an hydraulic press, it was possible to obtain the primary juice with around 55 % of the total removable fructans (FC), secondary juice (recovered from the pressed bagasse) with 32 % and tertiary juice (from the washings and pressing of the bagasse) with 12 % of the possible total fructans (Table 1). A second washing of the bagasse only allowed for the addition of 2 % of the total concentrate of fructans, which was considered unfeasible, having to evaporate water volumes similar to the previous juices.
Table 1. Fructan concentrate (FC) obtained (%) from each stage of the extraction method used, from the stems of Agave salmiana.
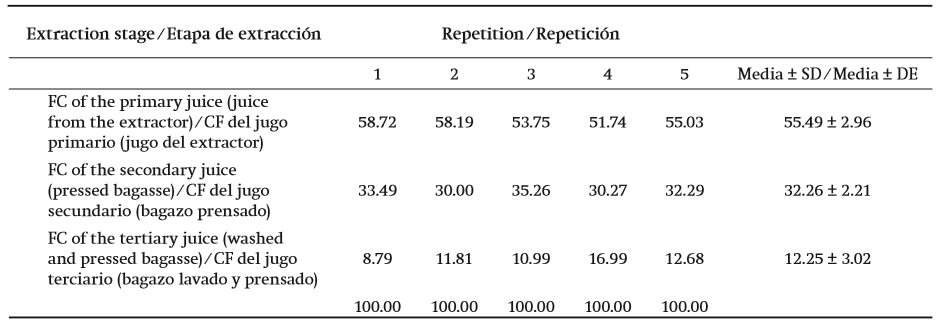
SD: Standard deviation
By using only the stem of the plant as raw material, it was not necessary to resort to filtration equipment (resins of ionic exchange, activated carbon, nanofiltration, etc.) and expensive processes to divide the pollutants that were present in the bases of the leaves (saponins, chlorophylls, waxes), as well as bi, tri and tetrasaccharides going through polymerization to be stored in the stem. The use of warm water as a solvent was enough to complement the extraction, as with the procurement process of inulin from the chicory stem (Niness, 1999), as well as being the most adequate in order to obtain a food product as natural as possible.
The pH of the primary, secondary and tertiary juices varied between 5.6 and 5.8; this slightly acid pH was higher than the one typically found in the foliar fluids of Agave species due to their type of metabolism (acid metabolism of the crassulaceae) (Medrano & Flexas, 2008). The pH plays an important role in the enzymatic reactions that happen on a cellular level in the plant, which continue during and after the extraction process, if they are not inhibited. In some works (Gupta, Kaur, & Kaur, 2003), the natural acidity has been neutralized with NaOH in order to inhibit the enzymatic activity; however in this study, in order to avoid chemical treatments, enzymatic deactivation was used with distilled water (which at the same time dissolves the fructans) at 55 °C for 60 min and the juices were frozen. These operations were critical as the probability of the enzymatic reactions continuity was very high (Van Arkel et al., 2014), even under refrigeration. Thus, the importance of extraction, stabilization and freezing of the juices on the same day of the collection of the maguey head.
The concentration of soluble solids of the primary and secondary juices of the stem varied between 25.0 and 28.8 °Bx, much higher values than 11.7 °Bx found in the raw juice of the entire head of A. salmiana (Moreno, 2013). This is due to the fact that with most fructans and dry matter content are found in the stem (Aguirre et al., 2001; Michel-Cuello et al., 2008), and when processing the entire head a dilution effect happens with the highest humidity of the stalk bases. The tertiary juice presented between 5.4 and 7.2 °Bx which mainly corresponds to remaining fructans, recovered with the washing and pressing of the bagasse.
The average estimated time to dry 12 L of juice (primary, secondary and tertiary) obtained from the stem of a 57.6 kg head, until reaching 95 % DM was 55 h. This time was much shorter than the average 480 h (20 days) required by Franco (2012) for the same purpose with only one air forced oven.
Absolute and relative yield of the fructan concentrate
As shown in Table 2, the stem of a maguey or head of 57.6 kg (less than 10 % of the total weight) yields an average of 1.05 kg of fructans with 95 % of dry matter. In addition, the leaf bases of this head could be used as fodder or to produce mezcal; thus, this yield allows us to assess the alternative use of the head of the maguey and its parts as raw materials.
Table 2. Absolute and relative yield of the fructan concentrate (FC) from the Agave salmiana stem.

SD: Standard Deviation
DM: Dry material
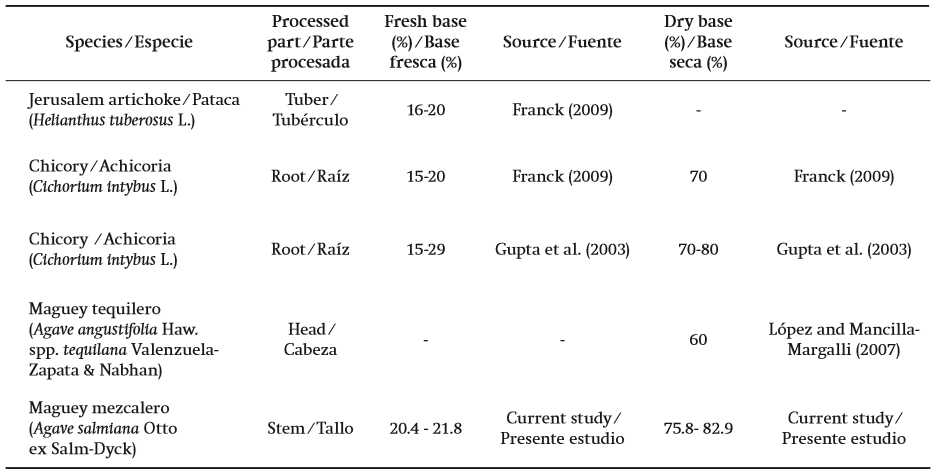
From the fresh stem of an average head of maguey mezcalero (57.6 kg), an average yield of FC (95 % DM) of 21 % was obtained, and regarding the dry weight of the stem, the yield was almost 80 %; both values are similar to the inulin content based on the fresh weight (15 to 20 %) and dry weight (70 to 80 %) of the chicory roots (Franck, 2002; Gupta et al., 2003). Table 3 compares the contents of fructans of A. salmiana and other species. The lower fructan yield (around 60 %) based on the dry weight of the head, documented by Mancilla-Margalli and López (2006) for sotol (specie of the Dasylirion genus) and different types of maguey, is expected when using the entire head as raw material, with all the aforementioned inconvenient events, in regards to the complexity of processes and quality of the extracted fructans.
Quality of the fructan concentrate
Table 4 presents the evaluation results of the mean quality of the FC (at a constant weight); that is to say, the relative content of extracted fructans from the stem of a ripe A. salmiana plant. As previously mentioned, an inulin standard was used as reference, as the equipment and the method prevent a higher resolution for fructans with a polymerization greater than level 5; therefore, the term inulin of this methodology truly corresponds to the maguey fructans (agavinas, according to López et al., 2014). It was found that the concentrate of the full extract of the second third of the stem has 78.31 % maguey fructans with more than five levels of polymerization, and that the glucose and fructose contents were not detectable (< 0.02 %). The composition of the fructan concentrate of the supernatant of the last third of the stem was similar to the composition of the full concentrate of the second third, even in its full recovery (95.03 %); both also presented a whitish appearance. In contrast, the composition of FC of the sediment of the last third of the stem was different even in its appearance, as it presented a dark brown coloration, greater total ash and sapogenins contents and a lower recovery percentage (79.31 %), maybe because it has unidentified or non-quantified components with this methodology. Regarding the weight of the total extract, the proportion of the sediment is only 5.6 %, so centrifugation could be a viable practice to more adequately purify maguey fructans.
Conclusions
The use of stems as raw material allows the implementation of simple and natural procedures for the extraction of maguey fructans. Through the pressing and washing of bagasse, the volume of FC obtained with an extractor can almost be duplicated. The yield of FC was 21 % of the fresh weight of the stem of a ripe maguey and around 80 % of the dry weight of the stem. The concentrate of maguey fructans, humidity free, presented a purity close to 80 % (fructans with more than five grades of polymerization). The alternative or complementary use of maguey mezcalero for the extraction of fructans, with a simple and natural extraction process, could stimulate the appreciation and use of the wild maguey plants, and at the same time, the rural development of the region.











 texto en
texto en