Introduction
Extractive mining is crucial to Chile's economy; however, it has resulted in a considerable number of abandoned mine sites, from small- medium- and large- scale mining. Some of these mining sites are now environmental liabilities, resulting in significant risks to the health of the population and the environment (Ginocchio et al., 2006; Karczewska et al., 2015; Mendez & Maier, 2008; Verdejo, Ginocchio, Sauvé, Salgado, & Neaman, 2015).
One of the minerals (or, from a biological perspective, micronutrients) of greatest importance in the soils of northern Chile is copper (Cu). It is an essential element for both animals and plants and has an important role in some physiological processes (Stern, 2010). Nevertheless, it becomes toxic at high concentrations (Canning-Clode, Fofonoff, Riedel, Torchin, & Ruiz, 2011). The improper handling of Cu wastes can lead to long-term and large-scale environmental impacts, which can be very difficult and expensive to remedy a posteriori (Ginocchio et al., 2004; Meier et al., 2012). There are various ways to rehabilitate areas with extensive environmental deterioration caused by pollutants generated by mine tailings. One of the mitigation techniques is known as phytoremediation (Environmental Protection Agency [EPA], 2000; Sarma, 2011), which comprises the use of plants to degrade, assimilate, metabolize or detoxify heavy metals and organic compounds in the soil (Azadpour & Matthews, 1996; Rascio & Navari-Izzo, 2011). Phytoremediation is based on naturally occurring processes by which plants and rhizosphere microorganisms degrade and sequester organic and inorganic contaminants. In fact, the most common approaches to the remediation of mine tailings are phyto-extraction (Nanda-Kumar, Dushenkov, Motto, & Raskin, 1995), hyper-accumulation of metallophytes (González, Muena, Cisternas, & Neaman, 2008), and phyto-stabilization using metallophyte phytoremediation techniques (Ginocchio & Baker, 2004).
Since the 1980s, re-vegetation has traditionally been used to stabilize solid waste and tailings, as well as to restore degraded areas, in order to prevent soil loss due to erosion and to rehabilitate the environment. However, international experience has shown that a more beneficial and effective method over the long term is stabilizing the tailings with phytostabilization (Li & Huang, 2015). Although phytostabilization may seem very similar to traditional re-vegetation, it has very different objectives and methodologies. Phytostabilization (Salt et al., 1995; Neuman & Ford, 2006) is the use of plants and remediators of a suitable substrate to immobilize or reduce in situ the bioavailability of metals in a solid substrate, such as mining waste (Li & Huang, 2015; Mendez & Maier, 2008). The implementation of a vegetative cover on the substrate also allows for physical stabilization, preventing dispersion of substrate metals by wind or water into surrounding areas. In addition, this approach obviates the need for chemical processing of waste. In Chile, despite the high diversity of species and the large number of existing mines, only some species of metallophytes for Cu: Schinus polygamus (Cav.) Cabrera, Atriplex deserticota Phil. (Ortiz-Calderón et al., 2008) y Oenothera affinis Camb. (González et al., 2008). Due to the importance of Cu and its role in soil pollution in northern and central Chile (Badilla-Ohlbaum et al., 2001; Ginocchio, Rodríguez, Badilla-Ohlbaum, Allen, & Lagos, 2002; González, 1994; Verdejo et al., 2015), it is critical that the adaptability of new vegetative species to extreme soil degradation conditions have been established. The objective was to determine and compare the phytostabilization capacities of native and exotic shrub species in areas highly damaged by mining activities.
Materials and methods
Study area
The tailings study was conducted around the Tunquén Mining Processing Plant, which is located in El Almendro, 25 km north of the city of Illapel in the Coquimbo Region, Chile (Figures 1a and 1b). It is located beside the Tunquén estuary at 550 m altitude. The tailings date from the mid-1980s (Figure 1c). The area covered by the tailings is approximately 4 to 5 ha at a height above the Tunquén estuary ranging between 20 and 30 m. The tailings were located within the Choapa watershed (31° 30' S lat. and 71° 6' W lon.), which is part of a predominately dry region with a low winter rainfall regime (Figure 1d). Annual rainfall ranges from 200 to 450 mm, with an average of about 240 mm in normal years (Favier, Falvey, Rabatel, Pradeiro, & López, 2009). Positive rainfall anomalies are observed during El Niño events, while below-normal rainfall is mostly associated with La Niña conditions (Falvey & Garreaud, 2007; Pizarro et al., 2012; Valdés-Pineda et al., 2014; Valdés-Pineda et al., 2015).

Figure 1. a) Study area located in the Coquimbo Region, Chile; b) Location of the mining plant; c) tailing area; d) Tailing area covered with plots containing the treatments.
The soil of the area is primarily a deep sandy-loam with predominantly moderately rapid permeability and very low organic matter content (Food and Agriculture Organization [FAO], 1998; Morales, Canessa, Mattar, Orrego, & Matus, 2006). The medium-hydrological soil type of this area offers favorable conditions for the formation of acidic water, which can infiltrate the soil and contaminate an entire water system; this contaminated water is in addition to that associated with the tailings dam. It is also obvious in this area that mining activity and high rates of wind erosion generate excessive particulate matter. In normal soil conditions, Cu concentrations range between 20 and 110 ppm (Pinto, Aguiar, & Ferreira, 2014). However, in soil solutions, Cu concentrations range between 0.03 and 0.25 ppm (Buccolieri, Buccolieri, Dell'Atti, Strisciullo, & Gagliano-Candela, 2010; Kwon-Rae & Owens, 2009; Mackie, Müller, & Kandeler, 2012).
Experimental design
For this study, two experiment periods were used. A total of 20 species (14 trees species, four evergreen shrubs and two herbaceous species) were used in the two experimental plots (Figure 2). Treatment "A" was planted in 2002 and consisted of a fertilized area, and an adjacent non-fertilized area. Individual species were planted in boxes of 40 x 40 x 40 cm at a distance of 2 x 2 m. An irrigation system was installed to provide 8 L of water per plant two times per day every three days. A total of 60 plants were established in treatment A, with three individuals of each species. Based on the results of the 2002 treatment, another plot was established in 2004 (treatment B). Only three species were used in this treatment with a total of 15 plants (five individuals of each species), and no fertilizer was applied. This three species were chosen because they showed the best survival rates in treatment A. Treatment B was irrigated at the same rate than treatment A. In addition, a control plot, treatment C, was also established.
Cu concentration of soils
The soil Cu concentrations from the treatment A and B plots were compared to soil Cu concentrations in the treatment C (control) plot by measuring Cu parts per million (ppm) at three soil levels 10-20, 40-50 and 60-80 cm with three samples taken at each level. We did not consider the 0-10 cm soil profile on these analyses since we assumed that Cu concentration is affected during the removal of soil that takes place when the plants are established. The only reason to measure Cu levels in each treatment plot soil was to show that they are, indeed, quite high, and that some species were able to survive and develop biomass at good rates in toxic levels of soil Cu. The total Cu concentration in soils was measured by atomic absorption spectrometry (Bersier, Howell, & Bruntlet, 1994; L'vov, 2005).
Survival rates and biomass evaluation
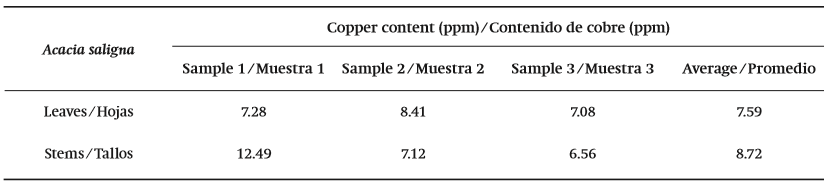
The data on individual plant survival were obtained from field campaigns made in January 2003 and December 2007 by the Corporación Nacional Forestal (CONAF). The evaluation of biomass development was performed using the following indices: Neck diameter (DC, mm), diameter of North-South canopy (DNS, cm), East-West crown diameter (DEO, cm), and height (H, cm). The total Cu concentration in plants was also measured by atomic absorption spectrometry (L'vov, 2005). The averaged Cu concentrations for each species were compared within each treatment, and between treatments. In addition, reference data about Acacia saligna (Labill.) H. L. Wendl. in normal soil conditions (Table 1) was compared to the Cu concentrations in the stems, leaves, and soil of A. saligna specimens at the treatment sites. Such reference content were analyzed by the research team, based on samples collected by the staff of the National Forestry Corporation of Chile (CONAF).
Statistical analyses
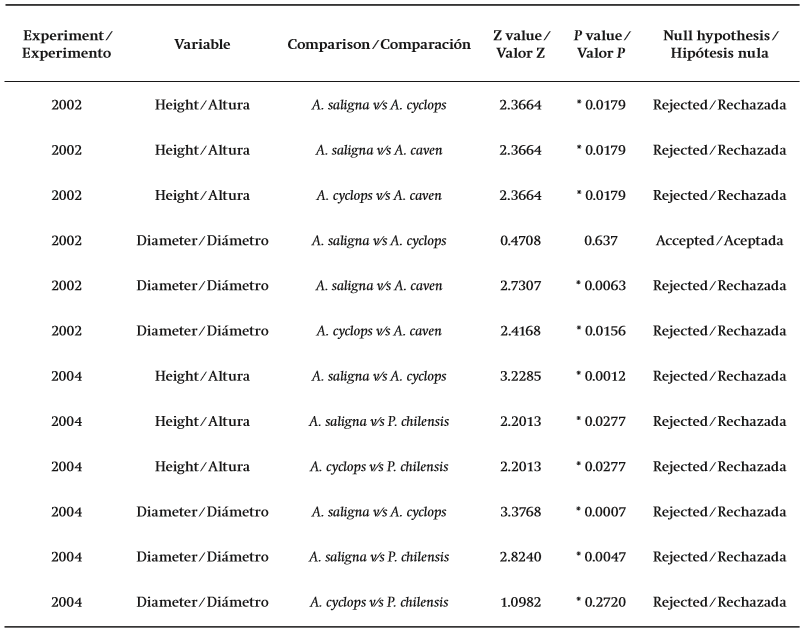
The non-parametric Kruskal-Wallis test (Kruskal & Wallis, 1952) was conducted to test the null hypothesis that medians within and between treatments are equal (H0 : x1 = x2). A confidence level of 95 % (P = 0.05) was used for all analyses and evaluations of biomass (heights and canopy diameters) and for Cu concentrations. To determine which concentrations were significantly different from the population median the Wilcoxon test was used, in which P values less than 0.05 lead to a rejection of the null hypothesis. The results were plotted and evaluated for all the individuals, species, and treatments, in order to determine the best species for phytostabilization activities in the study area.
Results and discussion
Survival rates
Despite the high concentrations of Cu observed in the tailings the species under analysis demonstrated good survival rates. For instance, the average survival rate of the species planted without fertilized soil (tailing area adjacent to treatment "A") was 74.8 %, while for species grown in fertilized soil (treatment "A") the average survival rate was 87 %. An average increase of 12 % in survival rates was observed for those species planted in fertilized soils compared to those in direct contact with the tailings. Only A. saligna, Atriplex nummularia Lindl. and Schinus molle L. showed 100 % survival rates in non-fertilized soils. These results were then used to evaluate biomass development.
Growth rates
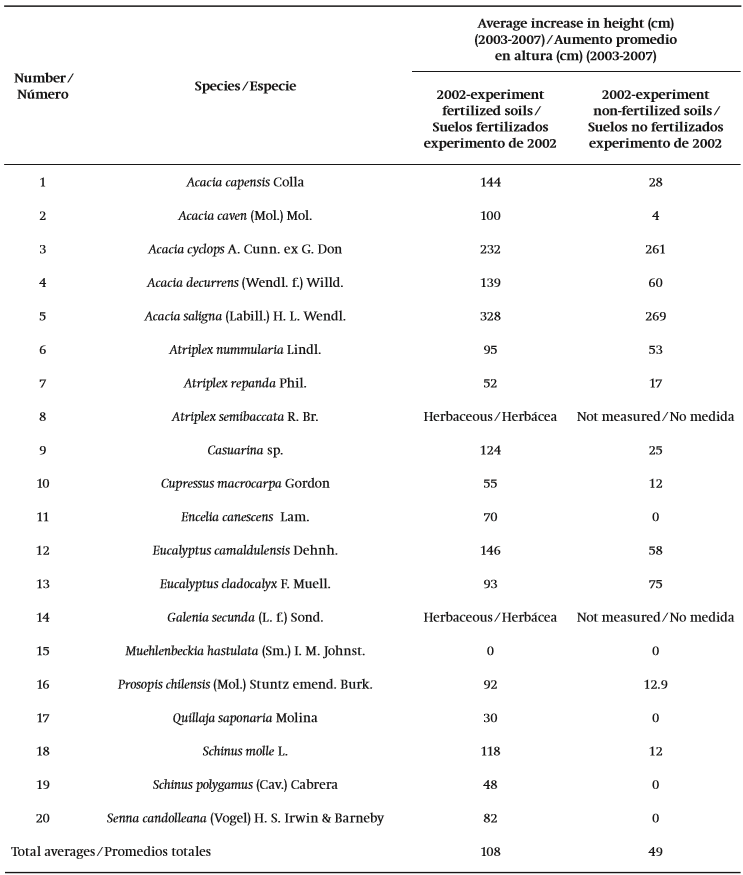
Assessing the average growth rates observed between 2003 and 2007, results indicated that A. saligna demonstrated the greatest levels of growth in both the fertilized and non-fertilized plots of the 2002 experiment, reaching 3.3 m and 2.7 m, respectively. Acacia cyclops A. Cunn. ex G. Don also showed notable height increases in the same period, reaching 2.6 m and 2.3 m respectively. Other species - for example A. capensis Colla and E. camaldulensis Dehnh. -- showed good results in fertilized soil, with both species reaching about 1.4 m (Table 2).
Table 2. Increases in height at the end of the measurement period (2007) for the 2002 experiment. Experiments established in Cu contaminated soils in the Coquimbo Region, Chile.
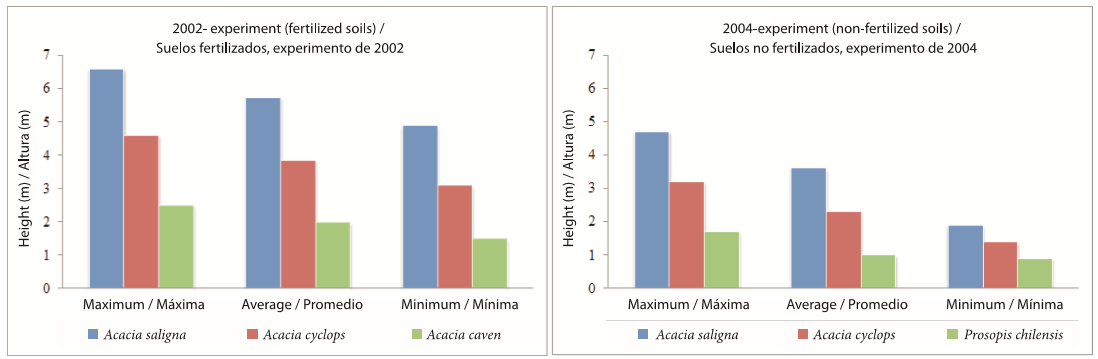
For the 2002 experiment, the individual specimens of A. saligna that had reached the greatest heights in 2007 were additionally measured in 2008, recording a maximum of 6.6 m and a minimum of 4.9 m with a coefficient of variation of 11.5 %. Acacia cyclops showed a maximum and minimum height of 4.6 and 3.1 m, with a coefficient of variation of 14.2 %. Acacia caven (Mol.) Mol. had the lowest heights -- between 2.5 and 1.5 m -- as well as greater variability in growth response (15.1 %) (Figure 3). The best responses observed for the 2004 experiment, indicated that, after only three years, A. saligna trees can reach heights between 1.7 and 4.7 m. Acacia cyclops reached heights ranging from 1 to 3.6 m; Prosopis chilensis (Molina) Stuntz was less developed in height (< 2 m) relative to the other two species mentioned (Figure 3).
Canopy diameter
The same species that performed well in terms of vertical growth showed the best results in terms of canopy development. For instance, in the 2002 experiment, A. saligna achieved the highest canopy diameters with a maximum of 4.9 m and a minimum of 2.5 m (Figure 4). The canopy areas for this species ranged from a maximum of 19.1 m2 to a mínimum of 4.8 m2. Acacia caven demonstrated less canopy growth: maximum canopy diameter was 3 m and maximum area was 16.9 m2, while minimum diameter was 1.4 m, and minimum area was 2.9 m2. In the 2004 experiment, the canopy diameter for A. saligna reached a maximum value of 3.9 m with a maximum canopy area of 11.9 m2. In this latter experiment A. cyclops and P. chilensis demonstrated the same pattern as the 2002 experiment, with much less development than A. saligna (Figure 4).
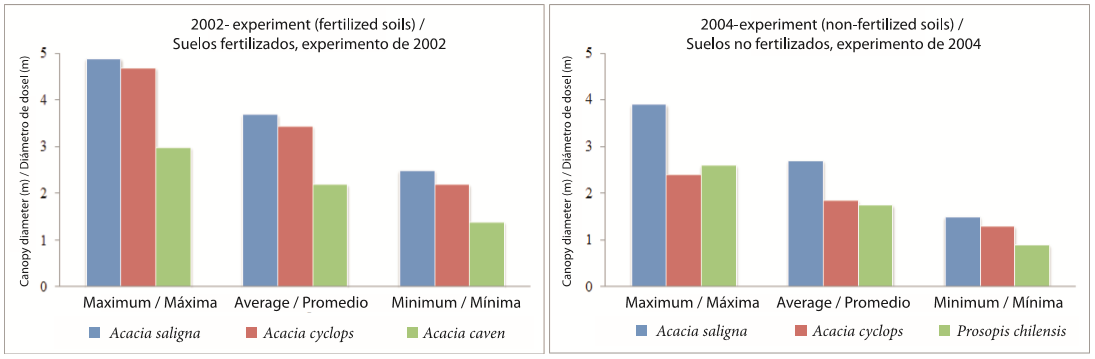
Figure 4. Variability in maximum, mean and minimum canopy diameter for individuals of Acacia saligna, A. cyclops and A. caven recorded in 2002 and 2004 experiments. Experiments established in Cu contaminated soils in the Coquimbo Region, Chile.
The statistical results and the final comparisons between the species regarding survival rates also demonstrated that the A. saligna trees are more adaptable in terms of growth and biomass development than the other species studied (Table 3; Figure 5). Similar results were found by Coates (2005) when studying selected tree species for a mine tailings bioremediation project in Peru. Coates' study showed that, of the 25 species and varieties tested, A. saligna had the largest base diameter and height. This study confirms that A. saligna is very able to adapt to unfavorable soil conditions with high Cu concentrations and in arid climates.
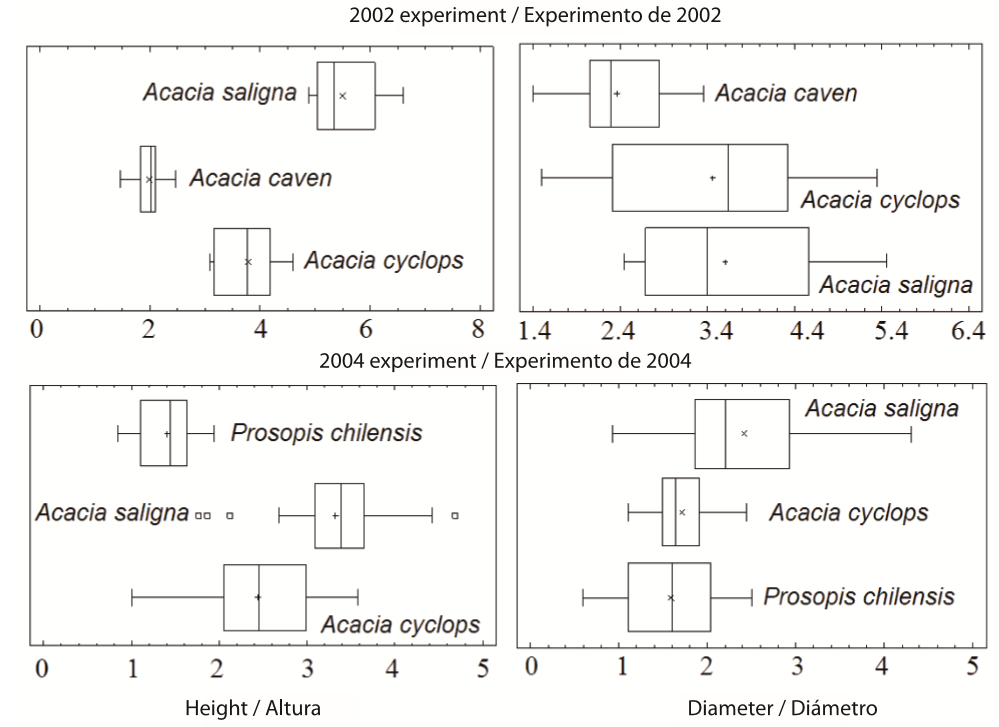
Figure 5. Box plot for the height and canopy diameter, for trees in the 2002 and 2004 experiments. Experiments established in Cu contaminated soils in the region of Coquimbo, Chile. The low quantile (25 %) and upper quantile (75 %) are represented by box limits; the median is represented by the line crossing the boxes.
Cu concentrations in soils and A. saligna
The average copper concentration at different soil depths (three samples for each depth at each treatment) showed higher concentration values closer to the soil surface (10-20 cm) with an average value of 3,064.8 ppm. Between 40 and 50 cm the average concentration was 2,045.2 ppm; and for 60-80 cm the average Cu concentration was 2,066.2 ppm. This pattern was observed within each treatment and between all treatments (Table 4). These Cu concentrations are larger than those found by Das and Maiti (2007) for Cu tailings located in India, which ranged between 1,008 and 1,803 ppm. Moreover these Cu concentrations range from two to more than 100 times larger than those found at 26 different agricultural soils in North-Central regions of Chile that were evaluated by De Gregori, Fuentes, Rojas, Pinochet, and Potin-Gautier (2003); they observed average Cu concentrations ranging between 11 and 530 ppm. The comparison of soil Cu concentrations in the treatments vs. control plots were not significantly different, indicating that trees did not absorb a significant amount of Cu or significantly rehabilitate the soil. The oldest trees in the study were only five years old at the time of the assessment, so it we hypothesize that the trees would absorb more Cu, and thus more meaningfully reduce soil Cu concentrations, as they become older and bigger.
Cu concentrations recorded in the leaves of A. saligna were significantly different from referenced values observed in Table 1 under normal soil conditions. Significant differences in the Cu concentration were only observed when comparing stem to leaf in the same individual within each treatment (2002 and 2004 experiments). Our results were found to be consistent with González et al. (2008), who indicated that the stems have significantly lower Cu concentrations than those observed in leaves (Figure 6).
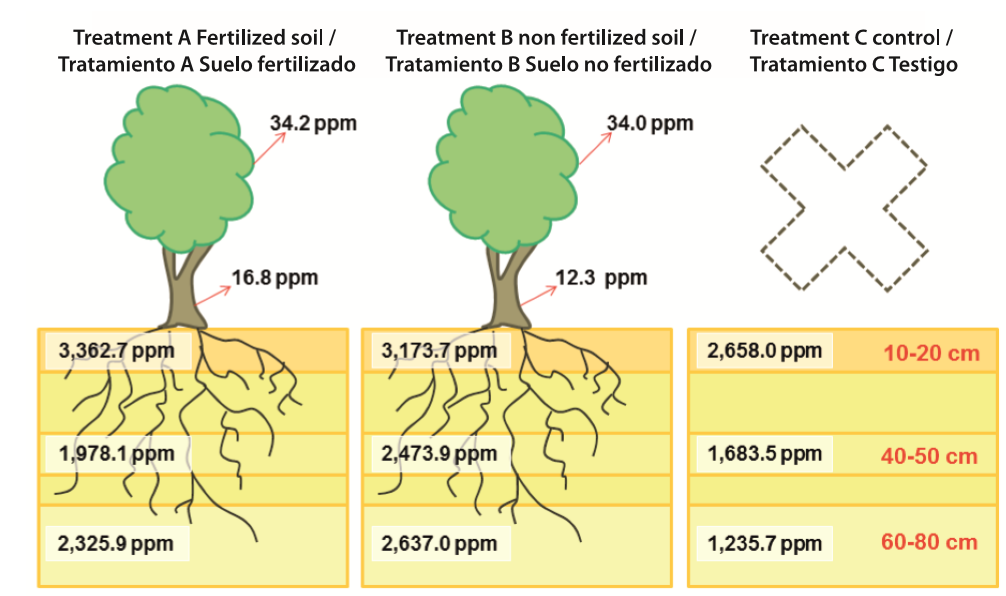
Figure 6. Conceptual representation of average Cu concentrations at each treatment, for stems and leaves of Acacia saligna (Treatment A with 15 samples and treatment B with eight samples); and for different soil depths (10-20, 40-50 and 60-80 cm) (each treatment with three samples).
Our results showed highest rates of Cu accumulation for both leaves and stems in A. saligna, when compared to all the species considered in this study. Thus, despite a large number of factors limiting growth and plant health -- such as fertility, physical, chemical and biological metal toxicity, and macro-nutritional deficiencies, among others - we demonstrate that the phytostabilization, for recovery of soils contaminated with copper, with native and exotic species is successful in the Coquimbo region. For these purposes A. saligna seems to be one of the best species, with higher survival rates and biomass development. This presents crucial information for future studies or phytostabilization projects aimed at minimizing the environmental impacts of mining or industrial activities in arid regions of Chile.
Conclusions
Various ways to rehabilitate mining waste areas are suggested in literature, but most of them are high-cost technologies with rapid deployment, and many are environmentally inappropriate. Within this framework phytostabilization techniques represent the cheapest approach with the least - and arguably a positive -- environmental impact. According to our results, A. saligna is the best species for phytostabilization activities in the Coquimbo Region. Despite these findings, it cannot be concluded that A. saligna should be the only species used, since a mix of different species (Cu accumulators) seems to be an appropriate alternative. Developing a mixed-species approach requires further research, in order to determine which combination of species and candidate trees achieves optimal forest development and rehabilitation of soils in areas contaminated by copper.











 texto en
texto en