Introduction
Mexico is the first producer of prickly pear worldwide; in 2020, it produced over two hundred fifty thousand tons (SIAP, 2021). The economic importance of this crop forces us to look for alternatives for its commercialization. The prickly pear is a fruit of the species belonging to the Opuntia genus. The fruit consists of two main parts: the pulp and the pericarp. The pulp is the edible portion of the fruit and constitutes 60-70 % of the total weight of the fruit. The major components of the prickly pear are sugar, fiber, mucilage, and pectins; proteins, amino acids, vitamins, and minerals are also present to a lower degree. It also contains antioxidants such as polyphenols and ascorbic acid as well as pigments such as carotenoids and betalains (Daniloski, et al., 2021).
The prickly pear pericarp (PP) represents about 50 % of the total weight of the fruit, predominantly non-cellulosic polysaccharides (about 40 %). PP tissues are mainly composed of parenchyma and collenchyma cells, as well as mucilage. Mucilage from prickly PP (MPP) is a complex polysaccharide of high molecular weight (2.3x104-3x106 g/mol) composed of L-arabinose, D-galactose, D-xylose, D-galacturonic acid, and L-rhamnose (Habibi et al., 2004).
The pericarp of prickly pear is a waste that may constitute a source of functional polymers and value-added compounds, particularly carbohydrates (Gheribi et al., 2019). However, taking advantage of both the pulp and the pericarp of prickly pear is an opportunity for the food industry.
The MPP is a hydrocolloid with the ability to retain water and create viscous or gelatinous colloids; it is also part of the diet fiber (Habibi et al., 2004). Studies on the use of nopal mucilage have applied this hydrocolloid as a thickener in fruit nectars (Sepúlveda et al., 2007) and edible films to protect fresh fruits and increase the shelf life of strawberries stored at 5 ºC. According to reports, the fruits maintained their texture, and flavor and prevented deterioration during nine days of storage. Medina-Torres et al. (2013) applied spray drying to encapsulate gallic acid, using an aqueous extract of MPP, which acted as wall material, showing the potential of this carbohydrate.
In recent years, there has been a growing trend toward the development of reformulated products by spray drying (Bhandari et al., 2013). This technique is well established in the food industry to transform liquids containing bioactive substances into stable solids (powders) of high quality. In addition, spray drying increases the stability of the product and makes it easier to handle, store and apply; this depends on the handling conditions, feeding characteristics, and design of the drier (Shishir & Chen, 2017).
For spray drying, the use of different materials known as stabilizers, adjuvants, vehicles, wall materials, or excipients is required. When spray drying is used as an encapsulation method, these polymeric materials have the function of protecting the encapsulated compounds. The materials are mainly polymeric with high molecular weights such as maltodextrins, these materials allowed to prevent. Stickiness is one of the main limitations of spray drying high-sugar food products such as juices (Fazaeli et al., 2012).
Some of the materials used in spray drying include polysaccharides (starches, maltodextrins, corn syrups, and gum arabic), lipids (stearic acid and mono- and diglycerides), and proteins (gelatin, casein, whey, soy, and wheat). Maltodextrin (MD) is a low-sweet polysaccharide consisting of D-glucose units mainly linked with α-1,4 bonds and obtained by acid hydrolysis of several starches (corn, potato, or others). It is highly soluble in water, has low viscosity and bland flavor, and produces colorless solutions. Therefore, it is widely used in the food industry (Sáenz et al., 2009) and microencapsulation.
The use of mucilage from some varieties of Opuntia as an encapsulating agent for betalain from various sources has already been tested. Mucilage has been reported to retain over 90 % of the betalain from Escontria chiotilla and Stenocereus queretaroensis fruits after three months of storage (Soto-Castro et al., 2019). The potential of Opuntia ficus-indica mucilage in the spray drying process is reported as well (León-Martínez et al., 2010). Also, there are studies in which the prickly pear is exploited for improving the antioxidant content (Gómez-Salazar et al., 2022). Furthermore, different encapsulation process was probed to protect the bioactive compounds in pear (Fernández-Repetto et al., 2023; Kurek et al., 2021). However, there is currently little documentary data describing the use of mucilage from prickly pears in spray drying (Otálora et al., 2015; Soto Castro et al., 2019).
In order to take advantage of the prickly pear pericarp as a source of MPP and evaluate its potential as a carrier in spray drying, we used MPP in combination with maltodextrin to spray dry prickly pear juice with the aim of obtaining high-added value products and offering a novel alternative for the consumption and marketing of prickly pear (Opuntia robusta Wendl. var. robusta).
Material and Methods
Prickly pear (Opuntia robusta Wendl. var. robusta) was obtained in Santiago Tolman, municipality of Otumba, Mexico state, and selected according to maturity degree. The soluble solids (≥ 12 ºBx) were measured with a digital refractometer (HI 96801, Hanna, Instruments, USA) with a 0-85 ºBrix scale and resolution 0.1 º Brix according to NMX-FF030SCFI-2006. The fruit was washed and peeled for juice extraction and mucilage.
Prickly pear juice preparation
Juice extraction was performed by crushing prickly pears without pericarp (PP) in a juice extractor to obtain two fractions: juice and pulp with seeds. Prickly pear juice (PJ) was sieved through 2 mm and 1 mm mesh to remove small pieces of pulp. It was then measured (ºBx) using a digital refractometer.
Extraction of mucilage from prickly pear pericarp (MPP)
The prickly pear pericarp was cut into 1 cm3 cubes that were placed in a stainless-steel container with water at a ratio of 1:2 p/p (PP/water). The mixture was heated at 80 ºC for 15 min (Medina-Torres et al., 2013). It was then filtered to recover the cooking water and remove the PP cubes. The cooking water was concentrated by heating it to 18-20 ºBrix. The concentrate corresponds to MPP and was mixed with four proportions of maltodextrin (MD) to encapsulate PJ by spray drying.
Spray drying of prickly pear juice with mucilage of prickly pear and maltodextrin
An experimental design was applied to mixtures of two components. The data analysis was performed with Design-Expert® v7.0.11 (Stat-Ease, Inc., Minneapolis, MN). Four mixtures of prickly pear pericarp mucilage/maltodextrin (MPP/MD) and control were obtained: P1 (50/50 %), P2 (33.3/66.6 %), P3 (25/75 %), P4 (20/80 %) and P0 as control (0/100 %). The mixture fed to the spray dryer had 16.6 °Brix with a 1:1 ratio of wall material (MPP/MD) and PJ. The spray drying process of PJ mixed with MPP and maltodextrin was carried out in a co-current flow spray dryer (Mobile Minor 2000, Gea Niro, Denmark). A two-fluid pneumatic nozzle was used at a spray pressure of 0.8 kg/cm2 while the inlet and outlet temperatures of the drying air were 180 and 80 ºC, respectively (Porras-Saavedra et al., 2015). The powders were kept in plastic containers in the dark at room temperature.
Product yield (powder recovery)
The encapsulation yield (EY) was determined as the percentage ratio between the total mass of the product recovered after spray drying and the mass of the extract fed to the system on a dry basis (Tontul & Topuz, 2017).
Characterization of powdered prickly pear juice
Determination of moisture content and water activity
Water activity (aw) was measured on a water activity meter (HBD5-MS2100WA, MAPISA, China) with temperature compensation and using the dew point method until equilibrium was reached. One gram of the powdered product was used at a temperature of 21 ± 0.01 ºC. The moisture content (g of water/100 g of sample) was evaluated by gravimetry. 3 g of sample were weighed and placed in an oven at 105 ºC until constant weight.
Determination of glass transition temperature (Tg)
The powder thermal properties were determined in a differential scanning calorimeter (Discovery, TA Instruments, USA) equipped with a reactor cooling system (RCS). The equipment was calibrated with indium (melting point 156.4 °C, enthalpy H = 6.8 cal/g); the purge gas used was dry nitrogen. 3 mg of samples (dry basis) were placed in aluminum pans. The heating program used included a heating rate of 10 ºC/min in a temperature range of -50 to 150 ºC (Zotarelli et al., 2017). The thermograms obtained were analyzed in TRIOS v4.3.1.39215 (TA Instruments).
Color determination
The color of the prickly powder was measured with a color reader (CR10, Konica Minolta) following the manufacturer's instructions. The CIEL*a*b* system was used. The reader indicated the L*, a*, and b* values. Determinations of each sample were done in triplicate and results were expressed as mean ± SD of three repetitions.
The color was evaluated by determining the color index (CI) obtained from Eq. (1)
Where L*, a*, and b* are the parameters of the CIELAB color system (Solórzano et al. , 2015).
Powder flow tests
Apparent and compact densities
The apparent density is the ratio of the mass of the untapped powdered sample to its volume, including the contribution of the space volume between particles. The apparent density was determined in 2 g powder weighed loosely in a graduated (10 mL) cylinder. The final volume was recorded, and the apparent density was calculated by dividing the sample weight by the volume (g/mL).
The compact density was obtained by following the same sample used in the apparent density evaluation without removing it from the cylinder. The cylinder opening was covered before testing. The cylinder was raised to a height of 10 ± 5 cm and tapped 250 times against a smooth, flat surface at a constant rate. The extracted volume (Vf) was recorded to the nearest unit on the cylinder scale. The casting density (g/mL) was calculated using the formula m/Vf where Vf is the final volume of the casting (Ortiz-Basurto et al., 2017).
Carr’s compressibility index and Hausner ratio
Carr's index and Hausner's ratio express the tendency of a powder to compress; They measure the compaction capacity of the powder and allow the relative importance of the interactions between particles to be evaluated. These indices were calculated with Eqs. 2 and 3, respectively:
Where CI is Carr’s index, Hr is the Hausner ratio, Vo is the bulk volume of the powder without tapping (mL), and Vf is the final tapped volume (mL).
Angle of repose
Five grams of the powder was poured onto a horizontal surface from a height of 10 cm using a funnel. The diameter and height of the formed cone were measured (Bhandari et al., 2013) to calculate the angle created (Eq. 4):
Where AR is the angle of repose, A is the height of the cone (cm), and r is the radius of the cone (cm).
Characterization by microscopy of prickly pear juice powers
Scanning electron microscopy (SEM)
The morphology of the powders was evaluated through images obtained by scanning electron microscopy (JSM-5800LV, JEOL). The samples were placed on slides with adhesive tape. Samples were sputter coated with gold on an ion coater for 10 min. Subsequently, images were obtained at different magnifications (1000x, 2500x, and 5000x) and a constant voltage of 15 kV (Porras-Saavedra et al., 2015).
Atomic force microscopy (AFM)
Atomic force microscopy (AFM) images of the samples were used to measure the topographical properties of the powders. A Di V Veeco multimode microscope was used. 2.5 x 2.5 µm 2 sections of each sample were analyzed in phase contrast in tapping mode (intermittent tip-to-surface contact) using an RTSP probe. To obtain the arithmetic mean roughness (Ra) and the root mean square roughness (Rq) of the images, image analysis was performed using the NanoScope Analysis 1.5 program (Pereyra-Castro et al., 2019).
Scanning laser confocal microscopy
To determine the distribution of PJ, MPP, and maltodextrin in the powders, a confocal laser scanning microscope LSM 710 (Carl Zeiss, Germany), equipped with lasers, was used. Autofluorescence images of all samples were obtained as follows: 405 nm at 4 %, 488 nm at 9 %, 561 nm at 2 %, and 633 nm at 2 % power; 40x magnification with an oil immersion objective (Porras-Saavedra et al. , 2015).
Determination of antioxidant capacity
The antioxidant capacity of the spray-dried PJ powers were determined using two different methods and concentrations of 2.5, 5, and 10 mg/mL.
Ferric reducing antioxidant
A mixture containing the sample (1 g), 2.5 mL of phosphate buffer, and 2.5 mL of 1 % potassium ferricyanide solution was incubated in an Isotherm® General Purpose Incubator (ESCO) at 50 ºC for 20 min. Then 2.5 mL of trichloroacetic acid (10 %) was added. The samples were centrifuged at 8000 rpm for 10 min. The supernatant (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1 % ferric chloride solution. The absorbance was read at 700 nm (Vijayalakshmi & Ruckmani, 2016).
Hydroxyl radical
The assay was performed against the hydroxyl radical (OH) produced from the Fenton reaction (Brands et al., 2019). The reaction was produced by mixing 1 mL of 1,10-phenanthroline, 2 mL of phosphate buffer (pH 7.4), 1 mL of FeSO4 (3 mM), 1 mL of H2O2 (0.12 %), and 1 mL of samples (2.5, 5 and 10 mg/mL), each of them dissolved in distilled water. The mixture was incubated at 37 °C for 90 min and the absorbance was read at 536 nm.
Quantification of betalains and betaxanthins
1 g of the sample was weighed and dispersed in 10 mL of distilled water. The suspension was vortexed for 1 min and then filtered through a 0.45 µm mesh, according to the method proposed by Coria-Cayupán et al. (2011). Betalain and betaxanthin content was measured in a spectrophotometer (6705 UV/visible, Jenway, Staffordshire, UK) at 536 and 480 nm, respectively, following the method of Stintzing et al. (2005). The extracts were diluted with distilled water until obtaining absorbance values of 0.9 < A < 1.0 to consider the maximum value. All determinations were performed in triplicate. Pigment content (mg/L) was reported according to the equation. (5):
Where A is absorbance, L is the cell length (1 cm), FD is the dilution factor, MW is the molecular weight (betalain 550 g/mol and betaxanthin 308 g/mol), and e is the molar extinction coefficient (betalain 60000 L/(mol cm) and betaxanthin 48000 L/(mol cm).
Determination of hygroscopicity
The hygroscopicity of the PJ encapsulates was determined according to the method proposed by Zotarelli et al. (2017). The powder (1 g) was weighed and placed in an airtight container at 25°C; a saturated NaCl solution was added to create an atmosphere of 75 % relative humidity. Seven days later, the samples were weighed and the hygroscopicity was determined; it was expressed in g of adsorbed water/100 g of dry solid.
Results and Discussion
Product yield (power recovery), moisture content, water activity, and glass transition temperature of spray-dried prickly pear
The highest product yield (PY) was P0 (Table 1), with a value of 78.2 %. Can-Karaca et al. (2016) reported that MD as wall material in spray-dried cherry juice allowed PY of 23-92 % depending on the operation conditions. This study aimed to evaluate the viability of the prickly pear shell as a source of mucilage to be able to use it in the spray drying of prickly pear juice. Therefore, the drying conditions were constant and the proportion of mucilage in the drying was varied. As the amount of MPP increased up to 50 % as wall material in the encapsulate (P1), the PY was reduced to 43.22 %. Samples P0, P2, P3, and P4 reached over 50 % of recovered powder showing a good recovery according to provisions of Tontul & Topuz (2017). Therefore, powders 3 and 4 containing MPP showing a similar recovery powder yield represent a good option for PJ spray drying.
The moisture content and aw increase as the MPP ratio increases in the sample (Table 1). Sample P1 with 50 % MPP had the highest moisture content (3.14 %) and water activity (0.34). The water absorption capacity of the MPP could be responsible for the trend of M% and aw. However, samples P0, P2, P3 and P4 presented M% below 5 % and aw < 0.30. These powders are considered microbiologically safe and can be stored for long periods, giving growers another option for marketing PJ.
The glass transition temperature (Tg) values are presented in Table 1. Tg values are within the range reported for spray drying of other juices such as mango (Tg of 32.4 ºC) using MD (10DE) as wall material (Zotarelli et al., 2017). The MPP content affected the Tg. As the content of MMP increases, the Tg decreases due to the presence of some sugars as glucose, fructose, and organic acids that have low Tg values (Roos & Karel, 1991). The Tg increases along with the crosslink density. MPP and MD are different types of polysaccharides; their structures can create interactions such as dipole-dipole and hydrogen bonds with molecules such as betalains and water, affecting Tg (Otálora et al., 2015).
Table 1 shows that powder P1 had the lowest Tg (8.98 + 0.43). As the MPP content decreased, the Tg increased and reached its highest value (Tg = 35.75 ºC) at P0. Yousefi et al. (2011) reported that Tg values > 40 ºC indicate a stable powder during storage and transport. According to their findings, a low Tg indicates a powder with high hygroscopicity, making it stickier (Yousefi et al., 2011).
Color of spray-dried powders of prickly pear juice
The color was measured and the CI was calculated from the L*a*b* parameters on powders. Table 1 shows the CI values obtained. Values range from -75 to -57. According to Vignoni et al. (2006), a CI of -40 is related to colors from blue to deep violet. The reduction in encapsulation CI is due to a decrease in the proportion of MPP added for spray drying. MPP contains pigments that are found in a smaller proportion when the amount of MPP in the formulation is reduced. ANOVA of CI corresponding to dry PJ samples using MPP and MD as wall material showed a significant difference between them, except for P4 (20 % MPP) and P0 (no MPP). This showed that adding 20 % of MPP to MD as wall material is not enough to modify the CI of PJ powder and obtain a significant difference. Otálora et al (2015) measured the color of spray-dried powdered betalain extract. They obtained values of L* 66.46, a* 29.99, and b* -4.84. Using these data, they found the CI to be -93.23, which is higher than PJ powder using MPP and MD as carriers. In our study, the entire PJ was used, so the betalain content was affected by the components of the juice.
Table 1 Effect of MPP on product yield, moisture, aw, Tg, and color of spray drying PJ.
| P1 | P2 | P3 | P4 | P0 | |
|---|---|---|---|---|---|
| Product yield (%) | 43.22 | 52.94 | 67.80 | 68.94 | 78.20 |
| Moisture (%) | 3.14+0.03c | 2.50+0.28ª | 2.96+0.36b | 2.46+0.32a | 2.12+0.26ª |
| Water activity (aw) | 0.34+0.01b | 0.26+0.02ª | 0.24+0.04a | 0.24+0.04a | 0.26+0.02ª |
| Tg (°C) | 28.98+0.43b | 30.09+0.79c | 31.46+0.79d | 32.07+0.5e | 35.57+0.66a |
| L* | 52.25 + 0.2b | 56.78+0.15c | 54.4+0.16d | 57.48+0.3e | 61.45+0.35ª |
| a* | +35.73+0.08b | +34+0.07c | +33.43+0.19d | +34.03+0.2e | +31.8+0.34ª |
| b* | -9.2+0.07b | -9.65+0.05c | -9.93+0.04d | -9.95+0.11e | -8.85+0.11ª |
| Color índex (CI) | -75.77d | -63.32c | -61.35b | -58.86a | -57.85a |
P1: prickly pear juice powder/mucilage in a 1:1 (v/v) ratio; P2: prickly pear juice powder/mucilage in a 1:2 v/v ratio; P3: prickly pear juice powder/mucilage in a 1:3 v/v ratio; P4: prickly pear juice powder/mucilage in a 1:4 v/v ratio; P0: prickly pear juice powder/without mucilage in a 0:1 v/v ratio. Different letters in the same line indicate a significant difference with p < 0.05 (n = 3 + standard error).
Flow properties of powdered prickly pear juice
Table 2 shows the flow properties of the powders. The apparent density range was 0.27 to 0.39 g/mL. The addition of MMP at 25 % allows us to obtain the highest apparent density and did not show a significant difference vs P0. Shishir & Chen, (2017) reported that bulk density depends on the size, shape, and surface properties of the particles. Powders with the smooth and uniform surfaces have a higher apparent density (Figure 1), so the packaging volume of the same quantity will be less. In general, apparent density increases as particle size decreases when more particles occupy a given volume. This allows for fewer spaces between the particles, which is desirable to reduce packaging and shipping costs (Tontul & Topuz, 2017).
Carr’s compressibility index and Hausner ratio
The Carr index was found in a range of 41.09-43.76 and Hr of 1.7-1.78 obtained in the five spray-dried PJ powders using MPP and MD (Table 2). The results show that the powders have low flowability and are highly cohesive. A variation in MPP concentration does not significantly affect these ratios versus only MD (P0).
Microstructural analysis of powdered prickly pear juice
Table 2 shows the size (diameter) of the spray-dried PJ. The diameter was found a range from 3.57 to 8.26 µm; the area ranged from 0.1 to 0.26 µm2. The smallest powder was P0 (MD only). The addition of MPP increased the size.
Medina-Torres et al. (2013) reported that the use of MPP in spray drying generates interactions between the MPP and the encapsulated compounds. This is favorable as it reduces the size of the aggregates. In addition, encapsulation with MPP as wall material is interesting since the chemical composition of the substance has some acid residues that give it a polyelectrolytic behavior.
From the results shown in Tables 1 and 2, the criteria for choosing the spray-dried prickly pear juice powder that had the highest MPP content and which showed the best characteristics for the powder with only maltodextrin as wall material. It was the P3 with 25 % MPP that allowed us to obtain particles with better characteristics in their techno-functional and surface properties.
Table 2 Effect of MPP on flow properties of spray drying PJ.
| Power flow test | P1 | P2 | P3 | P4 | P0 |
|---|---|---|---|---|---|
| Apparent density (g/mL) | 0.27+0.04b | 0.32+0.02a | 0.39+0.02a | 0.29+0.08a | 0.36+0.02a |
| Compact density (g/mL) | 0.48+0.07b | 0.57+0.02a | 0.72+0.08a | 0.50+0.2c | 0.64+0.03a |
| Angle of repose (AR) | 40.4 | 40.5 | 41.4 | 41.4 | 37.3 |
| Carr index (Carr I) | 43.76+1.41 | 43.70+3.9 | 43.54+2.8 | 41.09+1.4 | 43.03+3.2 |
| Hausner index (IH) | 1.78+0.04 | 1.78+0.13 | 1.78+0.11 | 1.70+0.05 | 1.76+0.1 |
| Area (μm2) | 0.247e | 0.262a | 0.143c | 0.168d | 0.100b |
| Diameter (μm) | 8.26e | 7.11a | 4.89c | 5.58d | 3.57b |
| Ra nm | 2.01+1.07b | 17.60+6.80a | 4.90+3.12c | 16.35+6.77a | 10.64+5.87a |
| Rq nm | 2.87+1.71b | 23.53+9.54a | 6.71+4.00c | 21.23+8.56a | 14.30+7.24a |
P1: prickly pear juice powder/mucilage in a 1:1 (v/v) ratio; P2: prickly pear juice powder/mucilage in a 1:2 v/v ratio; P3: prickly pear juice powder/mucilage in a 1:3 v/v ratio; P4: prickly pear juice powder/mucilage in a 1:4 v/v ratio; P0: prickly pear juice powder/without mucilage in a 0:1 v/v ratio. Ra: arithmetic average roughness. Rq: mean square roughness. Different letters in the same line indicate a significant difference with p < 0.05 (n = 3 + standard error).
Scanning electron microscopy (SEM)
Figure 1 compares images of PJ powders with MPP and MD. The particles show agglomeration with different sizes in a rounded shape with a smooth surface. Also, there is some large, cleaved particles. The formation of irregular surfaces in the particles obtained by spray drying is attributed to the contraction of the particles due to the inlet temperature during the drying process (Medina-Torres et al., 2013).
There were no differences regarding the content of MPP in the powders P2, P3, and P4. The powder P1 exhibited fused particles that maintained a round shape. Otálora et al. (2015) reported that the spherical morphology of the particles is a favorable characteristic in the powders obtained given the high surface tension of the molecular chains in the Opuntia mucilages after dehydration. This demonstrates its physicochemical potential as a material for the spray drying of food matrices with pigments and bioactive molecules such as PJ.
Atomic force microscopy (AFM)
Table 2 also shows the analysis of the images obtained by AFM. The arithmetic mean roughness (Ra), is the arithmetic mean of the absolute values of the ordinate roughness profile coordinates related to the midline within the measurement length and the root mean square of the roughness (Rq) of the PJ powder with MPP and MD. Ra values ranged from 2.01 to 17.6; P1 presented the lowest roughness and P2 the highest, no trend was observed for the values and content of added MPP. Analysis of variance revealed that there was no significant difference between P2, P4, and P0 which had the highest Ra and Rq, indicating that they had rougher particles.
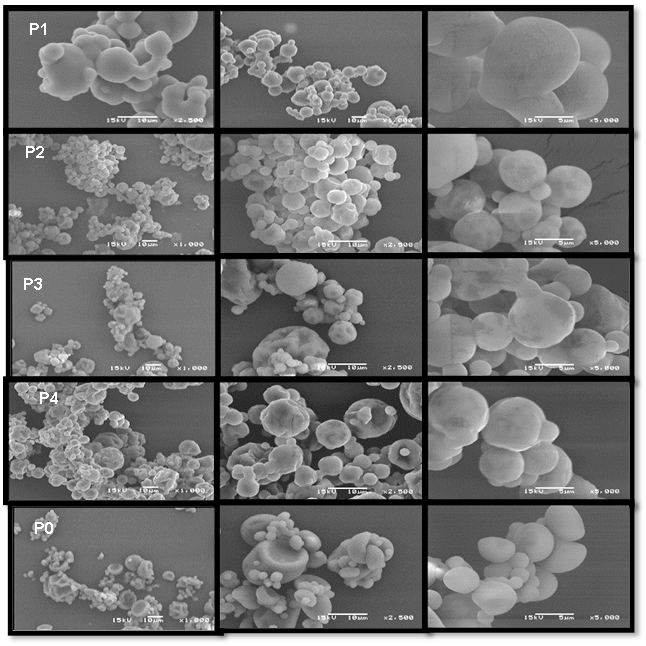
From left to right: Line P1 powder (mucilage and maltodextrin in a 1:1 ratio); Line powder P2 (maltodextrin mucilage ratio 1:2); Line powder P3 (maltodextrin mucilage ratio 1:3); Line powder P4 (maltodextrin mucilage ratio 1:4) and Line P0 (only maltodextrin). The images were taken at 1000x, 2500x, and 5000x magnifications.
Figure 1 Scanning electron microscopy images of spray-dried powders of prickly pear juice.
Figure 2 shows 3D and 2D topographic images of spray-dried PJ powder using MPP and MD in a sampling area measuring 2.5 µm. Acosta-Dominguez et al. (2016) reported that microencapsulates with a homogeneous surface and nanocavities < 15 nm had smooth surfaces. This increases the energy on the surface, which leads to a higher absorption capacity and a more even distribution of water and heat. They act as a physical barrier that reduces the mobility of water molecules, which could restrict the development of chemical reactions and prevent the deterioration of certain food components (Acosta-Domínguez et al., 2016).
The nanocavities of P2 and P4 were measured at greater than 15 nm and therefore were not considered for further determination. In contrast, those of P1, P3, and P0 measured below 15 nm; P0 was the control, and P1 (50 % MPP) showed the lowest Tg among the powders. It was selected P3 for further characterization because it has the MPP and allowed it to obtain particles with the best surface characteristics and techno-functional properties compared to P0.
Scanning laser confocal microscopy
Scanning laser confocal microscopy (SLCM) images of spray-dried PJ were obtained with MPP and MD in a 1:3 v/v ratio (P3). Excitation scans of the powder molecules at wavelengths between 450 and 700 nm and emission peaks revealed that MPP, MD, and PJ exhibit autofluorescence.
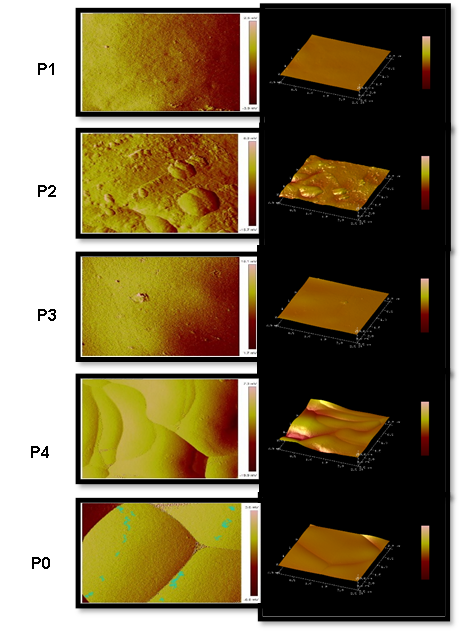
P1 powder (mucilage and maltodextrin in a 1:1 ratio); Line powder P2 (maltodextrin mucilage ratio 1:2); Line powder P3 (maltodextrin mucilage ratio 1:3); Line powder P4 (maltodextrin mucilage ratio 1:4) and Line P0 (only maltodextrin).
Figure 2 Atomic force microscopy images of the spray-dried powders of prickly pear juice.
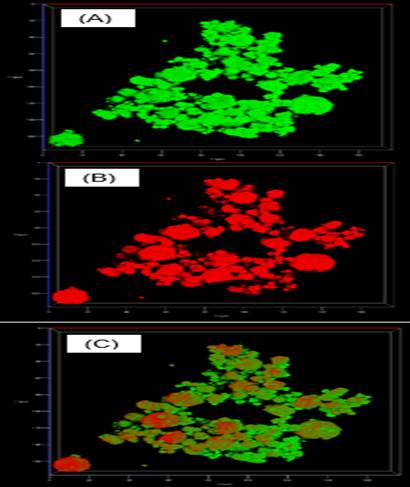
Figure 3 3D SLCM images of the P3 powder. (A) Prickly pear pericarp mucilage and maltodextrin; (B) Prickly pear juice; (C) Overlay of images (A) and (B).
Figure 3 shows the 3D SLCM images of the P3 powder. Image A) corresponds to the fluorescence of prickly pear pericarp mucilage (MPP) and maltodextrin (MD) in green, B) the fluorescence of prickly pear juice (PJ) in red, and C) are the superimposed images of the two components. In Image C, there are some red areas on the surface of some particles, mainly the larger ones, but not on the surface of the smaller particles. These images show the potential of MPP in combination with maltodextrin not only for juice drying but also for encapsulation and protection processes of the bioactive compounds of the juice. The smallest particles exhibit a uniform green color, corresponding to the MPP, which shows that the PJ is encapsulated.
The MPP used as wall material in combination with maltodextrin for the spray drying of the juice, allowed to obtain encapsulates with intense violet color, low moisture and aw, a smooth surface, small particle size, and a total encapsulation of the juice as shown by the different microscopies carried out, which favors its stability and possible use as an additive in the food industry.
Antioxidant capacity, betalains, and betaxanthins content
Table 3 shows that P3 presented an antioxidant capacity of 9.03 mM Trolox/L, more than double that of P0 (4.23 mM Trolox/L) determined by the reducing power assay. According to the OH radical assay, the inhibition of P3 is 38 % greater than P0.
Table 3 Antioxidant capacity, betalains, and betaxanthins content of spray drying PJ
| Test | P3 | P0 |
|---|---|---|
| Reductor power (mMTrolox /L) | 9.03 + 0.20 | 4.23 + 0.17 |
| Radical OH (% of inhibition) | 79.68 | 49.17 |
| Betalains (mg/kg SS) | 957.33+1.91 | 808.77+1.82 |
| Betaxantines (mg/kg SS) | 405.69+2.58 | 342.57+2.65 |
| Total of betalaines (mg/kg SS) | 1363.03 | 1151.35 |
P3: prickly pear juice powder/mucilage in a 1:3 v/v ratio; P0: prickly pear juice powder/without mucilage in a 0:1 v/v ratio. n = 3 + standard error.
Stintzing et al. (2005) determined the antioxidant capacity of different prickly pears in Trolox equivalents. The antioxidant capacity of purple prickly pear juice was 4.99 ± 0.37 mM Trolox/L, slightly higher than that of P0. Therefore, the antioxidant capacity value obtained in P3 was 44.7 % higher than that reported by Stintzing et al. (2005). This shows that MPP promotes an increase in the antioxidant capacity of the powder. Amaya-Cruz et al. (2019) evaluated the profile of bioactive compounds in red prickly pear pericarp. They identified 145 compounds: 68 extractable polyphenols, 15 hydrolyzable polyphenols, 41 betalains, 16 carotenoids, and 5 phytosterols. They suggest that MPP can be used as an ingredient to develop or improve functional foods.
The purple prickly pear contains betalains, which are classified according to their structure into betacyanins, responsible for the red-purple color, and betaxanthins, which provide the yellow-orange color. The total content of betalains in P3 and P0 are shown in Table 3. The content of betalains, betacyanins, and betaxanthins is higher in the MPP than in the PJ at 13.7 %, 19.45 %, and 6.69 %, respectively. The betacyanin content is about three times higher than that of betaxanthins. Garcia-Cayuela et al. (2019) determined total betalains (2400.0 mg/kg SS), betacyanins (1670.0 mg/kg SS), and betaxanthins (730 mg/kg SS) in purple Mexican prickly pear juice (red ball). The total content of betalains in the pericarp of the purple Mexican prickly pear was 1920.0 mg/kg SS; that of betacyanins was 1350.0 mg/kg SS while that of betaxanthins reached 570.0 mg/kg SS. When comparing these data with those obtained in this study, the total content of betalains in MPP was higher in the O. robusta prickly pear by 19.19 % compared to the red ball prickly pear, and in PJ it was lower by 17.71 %. This may be because the betalain content in prickly pear depends on the species, the crop, and the geographic region (Castellanos-Santiago & Yahia, 2008).
Plant mucilages have been used for many applications as stabilizers, emulsifiers, thickening or gelling agents, viscosity modifiers, encapsulating agents, and food packaging materials. In these studies, it has been reported that plant mucilages have the potential to extend the shelf-life of food products (Cakmak et al., 2023). Interestingly, in this research, the addition of MPP significantly increases (p < 0.05) the antioxidant capacity of the product. The P3 powder had a higher content of betalains, betacyanins, and betaxanthins than the P0, being 15.53 %, 18.36 %, and 15.55 %, respectively. The spray-drying process reduces the content of these bioactive compounds. However, P3 exhibited the highest antioxidant capacity compared to P0. The betalain reduction observed in P3 and P0 was mainly attributed to the drying process. The total retention of betalains for MPP and PJ was 59.56 % and 69.02 %. The betacyanin content was preserved. Although the pigments are diminished due to the spray drying process the powder retains a purple color as do MPP and PJ.
The MPP in the spray-drying of the juice allows for obtaining a product with good techno-functional properties and higher antioxidant activity compared to using only MD.
Hygroscopicity
The hygroscopicity of the P3 powder was 20.6 ± 0.37 g/100 g and 19.8 ± 0.12 g/100 g in P0 with a significant difference (p ≤ 0.05) between them. The hygroscopicity of the powder is generally related to its composition and particle size of the powder (O'Donoghue et al., 2019). The hygroscopicity values obtained in this study are lower than those reported by Zotarelli et al. (2017) for spray-dried mango pulp powder using MD. The authors obtained a value of 23.9 g/100 g, this difference between PJ powder and mango is probably due to the size of the particles and the sugar content (14-15 ºBrix) of the juices. Tontul & Topuz (2017) showed that powders with less than 20 % hygroscopicity are recognized as non-hygroscopic. So, P0 (only MD) is slightly hygroscopic, but P3 (MPP and MD) is hygroscopic, as it showed a value marginally higher than 20 %. However, to modify the particle size and decrease the hygroscopicity the drying conditions of the PJ with MPP and MD can be changed.
Conclusions
In this research, it was reported the potential of prickly pear pericarp mucilage for the spray drying process of prickly pear juice. The pericarp of the purple prickly pear (Opuntia robusta) is an agro-industrial waste and it can be used as a source of mucilage. The addition of mucilage decreased the yield and the glass transition temperature; increased the moisture and the water activity of powders obtained by spray drying. The addition of 25 % mucilage allowed us to obtain a powder with low moisture and water activity, a smooth surface, and a small particle size with an intense violet color, P3, the power that presented the best characteristics. This powder has the potential to be applied in the food industry. In addition, it offers an alternative to the marketing of tuna juice. The mucilage also increased the antioxidant activity of the powder compared to only maltodextrin.
For future research, it is suggested to evaluate the drying conditions to improve the yield and reduce the hygroscopicity of the powder. These findings promote the use of prickly pear pericarp as a source of mucilage for spray-drying prickly pear juice, offering an alternative for marketing and integral use of prickly pear.











 nueva página del texto (beta)
nueva página del texto (beta)



