Introduction
Onion (Allium cepa L.) is the most widely cultivated plant in the world, only behind potatoes and tomatoes, and is produced in more than 175 countries, where Asia contributes more than 60 % of world production. In the American continent, more than 9.7 million t of onion are produced. The United States, Mexico, and Brazil are the main producers of this vegetable. In 2021, Mexico produced 1,487,102 t in an area of 47,952 ha, where Chihuahua (330,371 t), Guanajuato (197,126 t), and Zacatecas (153,349 t) were the states with the highest production (FAOSTAT, 2021; SIAP, 2021).
Several factors negatively affect the production of this crop, phytonematodes being one of the most important, which cause annual losses in agriculture by 78 billion dollars worldwide (Agrios, 2005). The nematodes associated with onion crops are the stem and bulb nematode (Ditylenchus dipsaci), root-knot nematodes (Meloidogyne hapla, M. incognita, M. javanica and M. chitwoodi), the stubby-root nematode (Paratrichodorus sp.) and the lesion nematode (Pratylenchus penetrans) (Ravichandra, 2014; Mishra et al., 2014; Becker & Westerdahl, 2018). These nematodes can reduce bulb yield by up to 70 % if effective management tactics are not implemented. Marketable yields of onion were reduced by 31, 72, and 64 % with 2, 6, and 18 j2 of M. hapla per cm3 soil respectively (Merrifield, 1999). The bulb weight of onions in the M. incognita-infested microplots was 76 % less than in the root-knot nematode-free plots (Corgan et al., 1985). Bulb weight was reduced by 7 to 82 % and diameter by 10 to 62 % when plants were inoculated with 50 to 10,000 second-stage juveniles of M. graminicola. Onion bulbs from the field were reduced by 16, 32, and 35 % in weight and 6, 17, and 18 % in diameter when the percentage of roots galled was 10, 50, and 100%, respectively (Gergon et al., 2002).
In Mexico as well as in other countries, there are reports of Meloidogyne sp. in onion crops causing nutritional imbalance, stunting, and root galling (1-2 mm in diameter) in infected plants. Galls hinder the water and nutrient absorption in the plant causing growth retardation, reduction of plant vigor, and predisposing plants to be attacked by other phytopathogens (Becker & Westerdahl, 2018).
There is limited information on the effects of the root-knot nematode (Meloidogyne spp.) on onion crops in the Guanajuato state region, and given the importance of crops in this area, it is essential to understand this relationship and implementing management tactics that allow farmers to reduce the impact of these organisms on crops. In addition, root-knot nematodes are extensively controlled using chemical nematicides, predominantly broad-spectrum products or biocides such as 1,3-dichloro propene, chloropicrin, and methane sodium or non-fumigant nematicides from carbamates and organophosphates groups, which are highly toxic and affect the nervous system such as oxamyl, fosthiazate, aldicarb, and fenamiphos, many of them prohibited or restricted due to their toxicity to non-target vertebrates, mammals, and humans, exerting environmental impacts (Kearn et al., 2014).
Hence, it is necessary for more research and efficient assessment with an environmental approach to combat entomological threats with alternative products with less impact on the rest of the organisms that inhabit the soil. Among the alternatives, fluensulfone is a contact nematicide that belongs to heterocyclic fluoroalkyl sulfones, which are highly effective against Meloidogyne species although it is not yet evaluated in onion crops; furthermore, it exhibits more favorable toxicological features than conventional nematicides that are currently available in the market. Fluensulfone shows irreversible nematicidal activity and affects different nematode development stages and physiological processes in several routes; the toxicological and ecotoxicological profile is better than organophosphates and carbamate nematicides, commonly used to combat phytonematodes (Oka et al., 2009; Cabrera-Hidalgo et al., 2015). For example, the acute LD50 of fluensulfone via oral administration in rats is greater than 500 mg/kg, while for other popular nematicides such as aldicarb, fenamiphos, oxamyl, cadusafos, and fosthiazate; with an LD50 of 0.5-1.5, 2-19, 5.4, 37.1 and 73 mg/kg, respectively. In addition, it is not toxic to bees or earthworms (Oka et al., 2012), and due to its non-polar behavior, it exhibits low leaching in the soil due to its low solubility, such feature increases effectiveness periods against nematodes, with a soil half-life of 11-22 days (Oka et al., 2009; Kearn et al., 2014; Norshie, 2014).
Currently, within the group of non-fumigant nematicides, particularly carbamates and organophosphates, which were developed in the 50s, only oxamyl is still on record. Almost all of these substances were withdrawn from the market given their toxicological and environmental restrictions (Marbán-Mendoza & Manzanilla-López, 2012), and due to the low number of available nematicides and restrictions on the use of non-fumigant nematicides due to high toxicity to non-target organisms and to humans; fluensulfone is a potential candidate for use in integrated root-nematodes management programs in onion crops in Mexico; thus, the present work aimed to evaluate the effect of fluensulfone application on a Meloidogyne population in onion crop in field.
Material and Methods
Biological materials
Root samples in a naturally infested plot with root-knot nematodes in Romita, Guanajuato state were collected, while the diagnosis and identification of the species present in soil was carried out. Samples were processed for the extraction of juveniles and females. Twenty females were prepared to make perineal cuts according to the method described by Hartman and Sasser (1985) the morphology of the designs was compared with descriptions and illustrations by Hunt and Handoo (2009). Species identification was also carried out using the PCR technique by sequencing the 18S-rDNA (Holterman et al., 2006).
Nematicides
Formulations based on fenamiphos (Nemacur 400 CE®, 400 g a. i. L-1) and fluensulfone (Nimitz®, 480 g a. i. L-1) were evaluated. Fenamiphos is a systemic organophosphate with a broad-spectrum nematicide activity via direct contact and ingestion, interfering with the transmission of nerve impulses by inhibiting the enzyme acetylcholinesterase (IRET, 2021).
Effect of fluensulfone on the nematode population and crop damage
The effect of nematicides on a population of Meloidogyne sp. on cv. Cirrus onion plants in Romita, Guanajuato state were compared. The nematode population (Pi) was quantified before establishing the crop and before the application of treatments. Soil samples of 1.5-2 kg in each experimental unit (three sections of rows of 12 m x 0.75 m) were collected. Nematodes were extracted using three samples, each of 100 cm3 soil, using a sieving technique (Cobb, 1918).
Three fluensulfone (1.75, 2.0, and 2.25 L.ha-1) and one fenamiphos dosage (7 L.ha-1) were evaluated on juveniles of Meloidogyne sp. in cv. Cirrus onion plants. A non-treated control plot was included for comparison purposes. Fluensulfone was applied ten days before planting to the soil only once time, through an irrigation system. The furrows were moistened to field capacity and the product was subsequently applied. To incorporate the nematicide and to reduce the risk of phytotoxicity, irrigation was applied to the plots six days after application. Fenamiphos was applied at the time of sowing only once through the irrigation system. Onion plants were managed by the farmer, without applying any product with nematicidal action. Furrows without nematicide application were used as control treatments. The treatments were distributed under a completely randomized block design with three replicates.
The population density of nematodes in the soil (initial and final), reproduction rate, and root damage in onion plants at 30, 60, and 90 das, were evaluated; as well as the average bulb weight and estimated yield (g.m-2) at 100 das. The initial population (Pi) was evaluated before applying the treatments and the final evaluation (Pf) at 60 and 90 das, taking subsamples of approximately 200 g of soil (composite sample of 1.5-2.0 kg per experimental unit). The sample was homogenized and 100 cm3 of soil was used to extract the nematodes using Cobb’s sieving technique (Cobb, 1918). The reproduction rate (R) was determined by the equation R= Pf/Pi. Root damage was evaluated through a destructive sampling assay, galls were counted by visual inspection of the extracted roots, and then the scale proposed by Taylor and Sasser (1983) was applied. Moreover, the average weight of bulbs and onions yield at 100 das was determined. Plants were visually rated for leaf damage at 7 days after applying the product using the European Weed Research Society rating system (EWRS) (Champion, 2000).
The homogeneity of variance among treatments was verified through the Chi-Square test described by Bartlett (1937), and the normal distribution of the observations of each treatment was determined by the Shapiro-Wilk test (Shapiro & Wilk, 1965) with significance levels of p < 0.05. Obtained data were subjected to an analysis of variance and means comparison (Tukey, p ≤ 0.05). All statistical analyses were made with the software Statistical Analysis System (SAS) Version 9.3.
Results and Discussion
Morphological and molecular identification of nematodes
The population was identified as M. incognita based on morphological features according to Eisenback et al. (1983). The 18S-rDNA was sequenced to confirm the identity of the specimens. The perineal patterns agreed with the models and descriptions reported by Eisenback et al. (1983) for M. incognita which is characterized by an elongated shape with a high and squared dorsal arch, with usually wavy striae, and a lateral field is absent. The position of the excretory pore of females coincided with that of M. incognita (Figure 1). According to Karssen (2002), perineal patterns are widely used for identifying and describing Meloidogyne species since 1949, being one of the most important identification methods, since the perineal pattern is stable and does not change significantly during a prolonged culture period, females are relatively large, numerous, easy to find in infected tissues and suitable for preparing for microscopic examination, unlike males, which are difficult to find or juveniles since they are small, vermiform and difficult for preparing for microscopic examination (Eisenback & Triantaphyllou, 1991).
However, variations in the perineal patterns among individuals of the same species and non-standardized criteria of researchers when describing the perineal pattern, all these factors limit the precision of the identification at the species level using said patterns only (Hunt & Handoo, 2009). Species identification based on morphological features requires technical skills and time, which makes the process even more complicated. Therefore, sequencing of the 18S-rDNA region (accession MW699355) allowed us to corroborate the identity of the specimens. The 18S-rDNA region is highly conserved between species and has a slow evolution rate, and also exhibits enough variation and stability for the discrimination of the main root-knot nematode species (Roberts et al., 2016). The correct identification of these organisms has important implications in the design of integral programs on nematode management and is essential to successfully implementing combat measures in any agricultural production system. This allows a rational use of nematicides, reducing air and water pollution, and reducing negative effects on beneficial soil organisms.
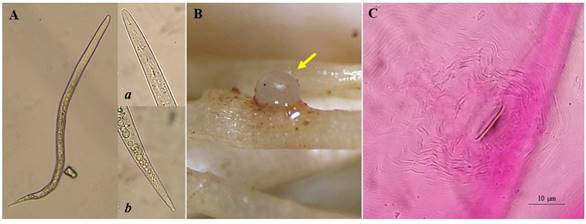
Figure 1 Morphological characteristics of the examined specimens. A) Second stage juveniles; a y b) Anterior and posterior region of a J2 respectively. B) Female of Meloidogyne incognita on onion root (yellow arrow). C) Characteristic perineal pattern of M. incognita females collected in Romita, Guanajuato associated with onion crop.
Effect of fluensulfone on the nematode population and crop damage
The average initial populations of M. incognita in the experimental plot fluctuated between 7.33 and 9.67 juveniles in 100 cm3 (Table 1) without showing significant statistical differences (p ≤ 0.05). Populations found in the present study are below the economic threshold indicated by Corgan et al. (1985), and Babu and Sivagami (1989), who indicated that population densities of M. incognita greater than 50 eggs and juveniles/100 cm3 of soil may cause significant losses of onion yield in sandy loam soils. According to Ferris et al. (1985) determining the initial population density of phytonematodes before establishing the crop is important as it allows for predicting the possible impact of nematodes on the growth and development of crops, as well as being an excellent tool for decision-making in the implementation of control measures.
Table 1 Effect of fluensulfone on the population of juveniles of M. incognita in onion crop cv. Cirrus at 30, 60, and 90 das. Romita, Guanajuato.
| Treatments | Pi | Pf (juveniles/100 cc of soil) | PD (%) ¶ | R | ||||||||||
| 0 | LS | 30 | LS | 60 | LS | 90 | LS | 30 | 60 | 90 | 30 | 60 | 90 | |
| FSF 1.75 | 7.3 | a | 3.7 | b | 10.3 | b | 16.0 | b | 73.8 | 56.4 | 42.9 | 0.50 | 1.4 | 2.2 |
| FSF 2.00 | 9.7 | a | 1.3 | b | 5.7 | b | 11.3 | b | 90.5 | 76.1 | 59.5 | 0.14 | 0.6 | 1.2 |
| FSF 2.25 | 7.7 | a | 1.7 | b | 5.0 | b | 12.7 | b | 88.1 | 78.9 | 54.8 | 0.22 | 0.6 | 1.6 |
| Fenamiphos | 8.7 | a | 2.0 | b | 9.7 | b | 11.0 | b | 85.7 | 59.2 | 60.7 | 0.23 | 1.1 | 1.3 |
| Control | 9.0 | a | 14.0 | a | 23.7 | a | 28.0 | a | 55.6+ | 163+ | 211+ | 1.56 | 2.6 | 3.1 |
| MSD | 9.34 | 5.52 | 6.63 | 9.84 | ||||||||||
MSD= Minimum significant difference. FSF= Fluensulfone. LS= Level of significance. Pi = Initial population. Pf = Final population at 30, 60, and 90 das. PD = Percentage decrease. R = Reproduction rate.
¶ Percentage decrease of the population compared to control treatment.
+ Percentage increase of the population in untreated plants, compared to the initial population.
* Means with the same letter in the same column are not statistically different (α= 0.05).
The population of M. incognita at 30 das showed significant differences (p ≤ 0.05), the bigger population in the untreated plots (14 juveniles/100 cm3 of soil on average) was found. Through the application of fluensulfone, nematode populations decreased by 73.8-90.5 % (3.7-1.3 juveniles respectively), when compared with untreated plants. Fluensulfone in a dosage of 2 and 2.25 L.ha-1 were the most effective to reduce the nematode populations (Table 1, Figure 2).
At 60 das, it was observed a slight increase in the phytonematodes populations in the plots treated with fluensulfone and fenamiphos, with average values of 5.0 to 10.3 juveniles in 100 cc of soil, which are statistically different from populations found in untreated plots (p ≤ 0.05) with more than 23 juveniles. The application of fluensulfone reduced the nematode populations by less than 80 % when compared to an untreated plot. Untreated plants showed an increase of 163 % in nematode populations with respect to the initial measured density (Table 1, Figure 2).

Figure 2 Temporal behavior of the Meloidogyne incognita population in onion crop cv. Cirrus. Romita, Guanajuato.
At 90 das, the behavior was similar to that observed at 60 days, the effect of fluensulfone the product decreased through time, with a reduction of less than 60 % with respect to control plants, in which populations increased by 211 % compared with the initial nematode density.
A single application of fluensulfone product (1.75-2.25 L.ha-1) before crop sowing effectively prevented the increase in nematode populations for up to 30 das, similar to a previous report by Cabrera-Hidalgo et al. (2015) with Nacobbus aberrans in greenhouse tomato and cucumber crops; and Norshie et al. (2016) in potato crop reported that fluensulfone applied to planting reduces Globodera pallida infection in potatoes roots, at least during the first 40 days. These results coincide with the observations made by Shirley et al. (2019) who reported reductions of 62 and 77 % in M. incognita populations in greenhouse tests with peaches 40 days after inoculation. This is possibly because fluensulfone has irreversible effects on nematodes reproduction, development, feeding, and mobility in a different way than the effect observed with organophosphates and carbamates which only paralyze them (Kearn et al., 2014), and therefore, the application of fluensulfone was effective in reducing the initial nematode populations, decreasing its reproduction rate, which opens the possibility of implementing it in integral nematode management programs, since fluensulfone is an eco-friendly product in contrast with organophosphates and carbamates that are commonly used for controlling phytonematodes (Cabrera-Hidalgo et al., 2015).
The reproduction rate of M. incognita was significantly suppressed with the application of fluensulfone, at rates lower than 1, thus affecting the nematodes reproduction by 85-91 and 75-78 % at 30 and 60 das, respectively, when compared with untreated plants (Table 1). The highest reproduction rate was observed in untreated plants with reproduction rates of 1.56, 2.63, and 3.11 at 30, 60, and 90 das, respectively. Nematodes reproduction is affected by several factors such as temperature, food availability, and agricultural practices, among others (Norton, 1978), as for the nematodes in untreated plots, without any critical limiting factors, such as nematicide application, the growth in a normal way showing the highest multiplication rates. On the contrary, in plots treated with nematicides, the reproduction of females was significantly lower when exposed to lethal and sublethal doses of the product, and according to Oka et al. (2009), nematodes that survived the nematicide could reach a reproductive stage, but then, fluensulfone could have inhibited egg hatching, as observed in M. javanica.
The analysis of variance of root galling showed significant differences among evaluated treatments (Pr>F<0.0001). A means test of (p ≤ 0.05) showed that the applications of fluensulfone (2.0 and 2.25 L.ha-1) and fenamiphos were effective to reduce the damage caused by Meloidogyne up to 60 das, with galling less than 15 % and control effectiveness above 80 % (Table 2).
At 30 das, damage caused by M. incognita in plants treated with fluensulfone and fenamiphos was 1.67-3.0 %, which represented a galling reduction of 89.5-81 %, respectively, compared with untreated plants, which presented the most severe damage with 15 % of galling (Table 2). No symptoms were detected in the aerial part of the plant; however, irregular bulges, necrotic injuries, and numerous small galls (< 2 mm in diameter) were observed in roots from infected plants, similar to that reported by Anamika et al. (2011) and Corgan et al. (1985).
Table 2 Root galling in onion plants cv. Cirrus caused by M. incognita at three evaluation moments. Romita, Guanajuato.
| Treatments | 30 das | 60 das | 90 das | Damage reduction (%) ¶ | |||||
| % | LS* | % | LS | % | LS | 30 | 60 | 90 | |
| FSF 1.75 | 3.0 | b | 15.3 | b | 24.5 | B | 81.0 | 53.3 | 54.6 |
| FSF 2.0 | 2.7 | b | 9.0 | c | 15.00 | C | 83.1 | 72.6 | 72.2 |
| FSF 2.25 | 1.7 | b | 6.3 | c | 11.50 | C | 89.5 | 80.7 | 78.7 |
| Fenamiphos | 1.8 | b | 6.7 | c | 12.00 | C | 88.4 | 79.7 | 77.8 |
| Control | 15.8 | a | 32.8 | a | 54.00 | A | 0.0 | 107.4+ | 241.1+ |
| MSD | 3.47 | 4.29 | 5.84 | ||||||
MSD= Minimum significant difference. FSF= Fluensulfone. LS= Level of significance.
* Means with the same letter in the same column are not statistically different (α= 0.05).
¶ Percentage decrease in damage compared to the control treatment.
+ Percentage increase in damage in untreated plants, with respect to damage at 30 das.
At 60 das, only fluensulfone application at a dosage of 2.25 L.ha-1 reduced root galling caused by M. incognita by 80.7 %. Fluensulfone (2.0 L.ha-1) and fenamiphos (7 L.ha-1) decreased damage by 72.6 and 79.7 %, respectively, compared to untreated plants. In the case of untreated plants, galling was 32.83 % on average, which represented an increase of 107 % compared to the damage observed at 30 das (Table 2).
At 90 das, root damage increased in treated plots (11.5-24.5 % galling), but was lower than the damage observed in untreated plants (54 % galling on average), where the galling increase was greater than 240 % when compared with damage at 30 das. The reduction of damage in treated plants was less than 80 %, showing the best response in 78.7 % with a fluensulfone dosage of 2.25 L.ha-1 with respect to untreated plants (Table 2).
Untreated plants exhibited growth arrest, leaf discoloration, and leaf tip burns (Corgan et al., 1985). An increase in the number and size of galls, thinning of infected roots, root distortion, and gall fusion were observed. Solitary and grouped galls (more than 10 contiguous galls) were observed (Fig. 3). Solitary galls were round 2-3 mm in diameter, and always accompanied by an exposed yellowish egg mass. Grouped galls were more elongated with similar diameters to lone ones, which did not exhibit the presence of egg masses; such types of galls are commonly formed by M. incognita. These symptoms coincide with those described by Mishra et al. (2014) and Parvatha (2014) in onion plants infected with Meloidogyne sp. A greater presence of purple roots was also observed in untreated plants, probably due to an infection caused by other pathogens (data not registered), since the attack of phytonematodes predisposes plants to infections by other organisms such as Fusarium, Rhizoctonia solani, and Thielaviopsis (Manzanilla-López & Starr, 2009; Parvatha, 2014; Ravichandra, 2014).

Figure 3 Root damage in onion plants cv. Cirrus caused by M. incognita. A-B) Galling and root thinning. C) Root galls with egg masses, c) Detail of egg mass. D) Purple root sections. E) Airborne symptoms in untreated plants (most affected area). F) General appearance on treated plants. Note the coverage and foliar coloration, and plant development in comparison with the untreated plants.
The present results suggest that a fluensulfone product applied before planting the onion crop can significantly reduce the populations and the damage caused by M. incognita in the soil and cv. Cirrus onion plants showed a better effect during the first 60 das; such effect may be due to the protection of the root system by inhibiting feeding and locomotion of phytonematodes treated with nematicides (Kearn et al., 2014). In addition, fluensulfone is capable of inhibiting nematode hatching and movement of nematodes (Kearn et al., 2014; Oka et al., 2009), which can reduce the inoculum load of nematodes in soil, and this could provide some protection to onion plants from damage associated with root infection.
Effect of nematicides on the production of onion cv. Cirrus
The onion crop yield at 100 das was evaluated with significant differences (p ≤ 0.05) found in the average weight of bulbs (AWB) (Table 3). The AWB fluctuated between 135.07 and 155.03 g. The highest weight was observed in treated plots with fluensulfone (2.0-2.25 L.ha-1) and fenamiphos (7 L.ha-1).
The estimated yield per m2 was higher in plots treated with fluensulfone (2.0-2.25 L.ha-1) and fenamiphos (7 L.ha-1) with 3360, 3431.1, and 3445.1 g.m-2 respectively, increasing the yield by an 11.9-14.7 % for untreated plants. Obtained production in treated plots with fenamiphos (7 L.ha-1) and fluensulfone (2.25 L.ha-1) were not significantly different (p ≤ 0.05), with more than 34 t.ha-1 of estimated yield, which represented an increase of 14 % compared to untreated plants (Table 3).
Table 3 The average weight of bulbs and onion yield cv. Cirrus at 100 days after sowing in Romita, Guanajuato.
| Treatments | AWB (g) | Estimated yield | |||||
| Average | LS* | g.m2 ¶ | LS* | T/ha ¶¶ | PI (%)1 | PD (%)2 | |
| FSF 1.75 | 145.1 | c | 3225.1 | c | 32.3 | 7.5 | 6.9 |
| FSF 2.0 | 151.2 | b | 3360.0 | b | 33.6 | 11.9 | 10.7 |
| FSF 2.25 | 154.4 | a | 3431.1 | ab | 34.3 | 14.3 | 12.5 |
| Fenamiphos | 155.0 | a | 3445.1 | a | 34.4 | 14.7 | 12.8 |
| Control | 135.1 | d | 3001.6 | d | 30.0 | 0.0 | 0.0 |
| MSD | 2.86 | 76.54 | |||||
MSD= Minimum significant difference. FSF= Fluensulfone. AWB= Average bulb weight. LS= Level of significance. PI= Percentage increase. PD= Percentage decrease.
* Means with the same letter in the same column are not statistically different (α= 0.5).
¶ Total weight of commercial bulbs per m2.
¶¶ Estimated yield considering a plantation density of 222,222 plants/ha.
1 Percentage increase in onion yield with respect to the untreated plot.
2 Percentage decrease in the onion yield of the untreated plants with respect to that obtained in the treated plots.
Yield was reduced by 6.9-12.8 % in untreated plants due to the damage caused by M. incognita to onion plants compared with treated plants. Such decrease, although low, is still significant, and is possible because the initial populations of the nematode were below the threshold reported by Corgan et al. (1985), and Babu and Sivagami (1989), suggesting that densities greater than 50 eggs and juveniles/100 cm3 of soil may lead significant losses of onion yield in sandy loam soils. However, if effective combat measures are not implemented, damages and losses could be greater in later crop cycles, due to the evident susceptibility of onion plants, since a cultivar is considered susceptible if it allows nematodes reproduction (Cook & Evans, 1987), which has serious practical implications, since M. incognita is cosmopolitan, polyphagous and exhibit high reproductive and survival capabilities (Hunt & Handoo, 2009), thus, said populations will rapidly increase causing more severe damages, since root-knot nematode feeding sites deform and block vascular tissues, limiting the translocation of water and nutrients in plants, with the consequent suppression of plant growth and yield losses (Mabrouk & Belhadj, 2012).
Finally, no visible symptoms of phytotoxicity in the evaluated dosage in onion crops were caused by the applied products, with values of 0.0-1.0 % of phytotoxicity according to the EWRS scale (data not shown).
Conclusions
Root galling associated nematodes in cv. Cirrus onion plants correspond to Meloidogyne incognita, based on the morphological features of juveniles and perineal pattern of mature females, and corroborated through molecular essays. When fluensulfone is applied, preventively protects the root system of onion plants for up to 60 das, increasing yields per m2 by up to 14 %. Onion crops and other economically relevant horticultural products in Mexico require a viable replacement for soil fumigants or an additional alternative to combat root-knot nematodes that may generate heavy losses in yields. The present work contributes in the right direction, in terms of providing producers with an efficient and ecologically viable management tool to combat root-knot nematodes, compared with the currently available synthetic nematicides.











 nueva página del texto (beta)
nueva página del texto (beta)


