Introduction
One of the most common diseases affecting chili seedling production in nurseries and greenhouses is known as “Damping off”. The phytopathogens causing such disease are a complex of fungi that include Fusarium spp, Rhizoctonia solani, and the oomycetes Pythium spp and Phytophthora capsici (González et al., 2013; Larios et al., 2019). This disease is considered cosmopolitan and one of the most important diseases in seedlings, causing losses of up to 100 %. Damage by this disease is observed in pre-emergence, post-emergence, and in the field after transplanting (Reveles-Hernández et al., 2010; Hernández- Hernández et al., 2018). Symptoms of this disease are; seed rot, hypocotyl necrosis, necrotic strangling at the base of the stem, root rot, wilting, and seedling death (Reveles-Hernández et al., 2010). For its control, farmers rely mainly on chemical fungicide applications (Captan, Metalaxyl, Azoxystrobin, among others), where low effectiveness and resistance of phytopathogens are reported, in addition to human toxicity and environmental contamination (Castillo- Reyes et al., 2015; Hernández- Hernández et al., 2018; Larios et al., 2019).
An alternative management option for plant diseases is biological control through the application of antagonistic microorganisms (Hernández- Hernández et al., 2018; Larios et al., 2019), which include several species of Bacillus spp, Pseudomonas spp, Trichoderma spp, among others (Asaka & Shoda, 1996; Gravel et al., 2005), which are not harmful to health and the environment (Larios et al., 2019), even do not promote resistance (Espinoza-Ahumada et al., 2019). These microorganisms act by different modes of action, such as parasitism, competition for space and nutrients, synthesis of antibiotics, or induction of systemic resistance in plants (Chirino- Valle et al., 2016; Espinoza-Ahumada et al., 2019), in addition, some genera are considered as plant growth promoters (Sivasakthi et al., 2014). Among the species that are known for their high capacity to produce low molecular weight antifungal compounds, the species Pseudomonas donghuensis stands out, which produces five times more siderophores than the other species, being this species a good option for phytopathogens control (Gao et al., 2015), since it has shown to have an antagonistic effect against several phytopathogens (Ossowicki et al., 2017). Therefore, this work aimed to test the effectiveness of bacterial isolates (Bacillus subtilis and P. donghuensis) to inhibit the growth of the causal agents of “Damping off” of chili, as a biological alternative for disease management.
Material and Methods
Experiment location
The research work was carried out in the Microbiology Laboratory of the Department of Agricultural Parasitology of the Universidad Autónoma Agraria Antonio Narro (UAAAN) located at Calzada Antonio Narro 1923, Buenavista, 25315 Saltillo, Coahuila, Mexico.
Biological material
The strains of the antagonistic bacteria were kindly provided by the Microbiology strain of the Parasitology Department of the UAAAN, which consisted of three isolates of the genus Bacillus spp (B15, BITV, and BIBT) and one isolate of Pseudomonas sp (Pd) recovered from the root of zacatón (Muhlenbergia macroura). The isolates were reactivated and purified on Nutrient Agar (AN) and incubated at 28 ± 2 °C for 72 h.
Bacillus spp and Pseudomonas sp isolates were molecularly identified based on 16S rDNA gene sequencing. The genes were amplified using a Verity end-point PCR thermal cycler (Applied Biosystems) (Jang-Jih et al., 2000) at the facilities of the Laboratorio Nacional de Biotecnología Agrícola, Médica y Ambiental (LANBAMA) located in San Luis Potosí, Mexico. In addition, the species had already been characterized morphologically by compound microscopy and by biochemical tests.
The phytopathogens isolation was performed from samples of chili serrano seedlings 30 days old, with symptoms of “Damping off” and coming from greenhouses in the agricultural region of Parras de la Fuente, Coahuila, Mexico, during the summer of 2019. For isolation of phytopathogenic fungi, substrate residues were removed from the root ball, the whole plant was washed with potable water under aseptic conditions, and small pieces of roots and stems were cut with a sterile scalpel and disinfected in a 1 % NaClO solution for 3 min, washed three times with sterile distilled water for 1.30 min and allowed to dry inside a laminar flow hood on sterile stratified paper. Once dried, they were transferred to Petri dishes with Potato-Dextrose-Agar (PDA) or V8-Agar (V8-A) culture medium and incubated at 26 ± 2 °C for 3 days. When mycelial growth was observed, it was transferred to Agar Water (AA) for 48 hours and purified by the hyphal tip in Petri dishes with PDA and V8-A.
Pathogenicity test
The technique used by Sánchez et al. (1975) and modified by Espinoza-Ahumada et al. (2019) was used, for which 50 seeds of chili hybrid Platino were germinated in AA medium, disinfected for 3 min in a 1 % NaClO solution and washed in sterile distilled water three times. Five days after sowing, once the hypocotyl had developed, they were transferred in groups of three seeds to Petri dishes with an AA culture medium. When root and cotyledon development was observed (two days later), a culture explant 3 mm in diameter with the mycelium of the purified phytopathogen was inoculated in the center of the Petri dishes. Petri dishes were incubated at 26 ± 2°C with a photoperiod of 12:12 (light: dark). To determine pathogenicity, seedling mortality was evaluated for nine days using a visual scale of severity described by Apodaca et al. (2004) with modifications, where 0 = no symptoms, healthy plant, 1 = small necrotic spots on the root or cotyledons, 2 = necrosis at the base of the root, 3 = root necrosed up to 50 %, 4= root or plant completely necrotic. The data obtained were analyzed through an analysis of variance (ANOVA) to stratify the treatments through a comparison of means by Tukey (p ≤ 0.05) and under a completely randomized design with two treatments (1 = P. aphanidermatum, 2 = F. oxysporum), absolute control and three replicates.
Determination of in vitro antagonism
The test was carried out by the dual confrontation technique between antagonist and phytopathogen in plates with Potato Nutrient Broth Agar (ANCP), for this purpose, the plates were marked in four cardinal equidistant points, in each one a roast of each antagonist strain was placed, after 24 or 72 hours a disk-shaped explant with mycelium of each phytopathogen was placed separately and incubated at 26 ± 2 °C until the mycelium of the control filled the culture plate. The experiment was conducted under a completely randomized design with 4 treatments (B15, BITV, BIBT, and Pd) and a control, with 4 replicates per treatment for each phytopathogen (Fusarium and Pythium). Radial growth of the phytopathogen (mm) in confrontation with each bacterial strain was measured, and this was transformed to percentage inhibition (%I), using the formula %I= (C-T)/Cx100 described by Castillo- Reyes et al. (2015), where C is the diameter of the control and T is the treatment. Data were subjected to analysis of variance (ANOVA) and Tukey’s mean comparison test (p ≤ 0.05), with the statistical program InfoStat version 2019.1.2.0.
Results and Discussion
Identification and characterization of Bacillus spp and Pseudomonas sp.
Bacterial species were identified based on their 16S rRNA gene sequencing. Species of the genus Bacillus named B15, BITV, and BIBT showed 91.91, 98.36, and 96.15 % similarity to B. subtilis species with GenBank accession keys of MK616213.1, MH619505.1, and KY010584.1, respectively. Although strain B15 coincides with a percentage of 91.91 % with the species B. subtilis, this percentage is considered low, in addition, this strain in previous studies presented a higher percentage (94.12 %) of similarity with the species Bacillus pumilus when morphological and biochemical tests were performed (Ordaz, 2004), thus the morphological and biochemical identification is more acceptable. The three strains (B15, BITV, and BIBT) presented a typical form of double bacillus with endospore production (Figure 1A, B, and C), Gram+ staining, and positive catalase (Sosa et al., 2005).
The bacterium of the Pseudomonas genus showed a 96.12 % similarity with the species Pseudomonas donghuensis with access code MK883145.1. This bacterium isolated from the root of M. macroura and by streak sowing in phosphate agar (NBRIP) (Flores et al., 2014) presented a yellow translucent colony formation characteristic of the genus Pseudomonas spp. In the beginning, the isolation presented irregularity to solubilize tricalcium phosphate. This bacterium presented itself as an individual vegetative bacillus or in pairs, non-sporulating (Figure 1D), Gram- (Figure 1E) with polar flagella and positive catalase. In AN at 48h of incubation, their colonies generate a yellow pigment (Figure 1F), while in King B medium they do not produce fluorescence and their colonies show irregular borders. Gao et al. (2012) first reported this species of Pseudomonas, later named Pseudomonas HYS, Gao et al. (2015) classified it as a new species named P. donghuensis isolated from the water of Donghu Lake of Wuhan in China, other researchers report its isolation from tomato rhizosphere (Ossowicki et al., 2017), cotton (Tao et al., 2020) and agricultural soil (Ágaras et al., 2018). The traits observed by P. donghuensis isolated in this research are similar to those described by Ágaras et al. (2018) for the species P. donghuensis SVBP6, but there are differences with respect to those reported by Gao et al. (2015) which pointed out that P. donghuensis HYS develops fluorescence on King A and B agar, while the strain in the present study did not produce fluorescence on King B medium, which coincides with Ágaras et al. (2018).
Identification of the species responsible for “Damping off”.
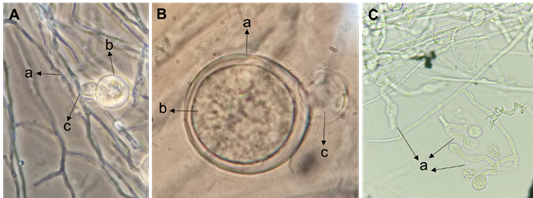
A fungus and an oomycete responsible for “Damping off” were isolated from chili seedlings. The phytopathogenic fungus showed septate hyphae, globose chlamydospores (Figure 2Aa), short phialides (Figure 2Ab) where kidney-shaped bicellular microconidia are inserted (Figure 2Ba), curved canoe-shaped macroconidia of three (Figure 2Bb and Cb) or four septa (Figure 2Ca), with foot cell (Figure 2Bc), besides the formation of sporodochium (Figure 2D). These observed microscopic morphological characteristics corresponded to the genus F. oxysporum according to the specialized taxonomic keys of Leslie and Summerell, (2006).
The identification and report of the oomycete corresponded to Pythium aphanidermatum, such work was published before the present research and was reported as an aggressive and fast-growing strain that causes “Damping off” (Jiménez-Pérez et al., 2022). The hyphae were ascertained to be coenocytic toruloid (Figure 3Aa), smooth spherical terminal oogonia (Figure 3Aa, Ba), aplerotic oospores (Figure 3Bb), diclinous or monoclinous antheridia (Figure 3Ac, Bc), and filamentous and irregular sporangia (Figure 3Ca), as shown in Figure 3 (Van der Plaats-Niterink, 1981; Watanabe, 2010; Jiménez-Pérez et al., 2022).
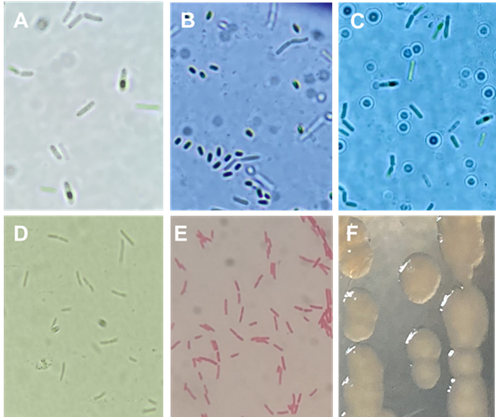
Figure 1 Morphological characteristics of Bacillus subtilis and Pseudomonas donghuensis. A, B and C) vegetative cells and endospore production of B. subtilis (A- B15, B- BITV and C- BIBT). D) P. donghuensis vegetative cells, B) Gram stain. C) Bacterial colony in AN culture medium.
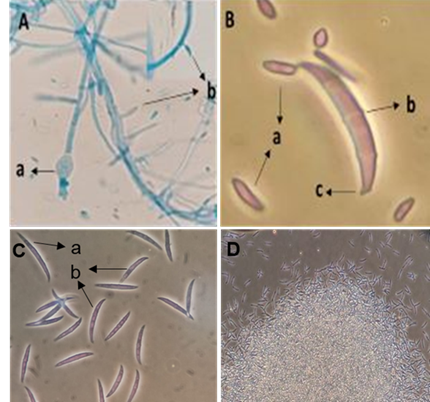
Figure 2 Microscopic observation of Fusarium oxysporum. A) a- Chlamydospore globosa and b- Phialides, B) a- Microconidia, b- Macroconidia with three septa and c- foot cell. C) a- Macroconidia with four septa and b- with three septa. D) Sporodochium.
Pathogenicity test
In the pathogenicity test, chili seedlings were inoculated with culture explant with mycelium of F. oxysporum (Figure 4B) at four ddi caused necrotic spots on the root, which developed and grew until completely necrotizing the seedlings at nine ddi, in addition, abundant growth of white mycelium was detected that completely covered the seedlings, causing 100 % mortality of the seedlings (Figure 4E). In chili seedlings inoculated with P. aphanidermatum (Figure 4C), at three ddi they showed necrosis, roots colonized by mycelium, cotyledons with necrotic spots, and at five ddi the seedlings showed abundant mycelial growth, causing 100 % mortality of the seedlings (Figure 4F). In controls, normal root, stem, and cotyledon growth were observed, with no signs or symptoms of necrosis (Figure 4A-D). According to the severity scale of Apodaca et al. (2004), the level of damage observed in the cotyledons, hypocotyl, and root of seedlings inoculated with P. aphanidermatum and F. oxysporum is the highest level (4), since they caused the death of the seedlings, while in the controls there was no damage. Therefore, it is suggested that P. aphanidermatum and F. oxysporum species were the causal agents of “Damping off” and death of chili seedlings.
The results of this study coincide with the reports of other authors on the ability of these phytopathogens to cause “Damping off”, such as Gravel et al. (2005) who reported P. aphanidermatum and P. ultimum as etiological agents of “Damping off” and seed rot in tomato seedlings, as well as González et al. (2013), who report the phytopathogens Pythium and Fusarium in tomato seedlings. While Sánchez et al. (2015) report F. oxysporum as the causal agent of “Damping off” in onion seedlings. Similarly, Rivera-Jiménez et al. (2018) reported that F. oxysporum caused the death of 49.54 % of chili poblano seedlings in 30 days of the test under greenhouse conditions, in contrast to the present work that was performed under in vitro conditions and where higher mortality of seedlings in less time (9 ddi) was observed.
Determination of in vitro antagonism
In the confrontations of F. oxysporum with Bacillus subtilis and P. donghuensis strains, a significant statistical difference (p ≤ 0.05) was observed in the percentage of inhibition. All Bacillus strains showed inhibitory capacity, with B. subtilis (BITV) having the highest efficiency in inhibiting mycelial growth of F. oxysporum, at 56.24 % (Figure 5C), followed by B. subtilis (BIBT) with 49.62 % (Figure 5A) and the B. subtilis isolate (B15) with the lowest inhibition (39.35 %) (Figure 5B). The P. donghuensis strain (Pd) did not inhibit the growth of F. oxysporum (Figure 5D) during the six days of the experiment, which is the time of the control to grow on the entire culture plate (Figure 5E) (Table 1).
Table 1 Percentage of in vitro inhibition of the treatments against Fusarium oxysporum six days after the confrontation.
| Treatments | ||
|---|---|---|
| Strain | Code Id | Means |
| Bacillus subtilis | BITV | 56.24A |
| Bacillus subtilis | BIBT | 49.62B |
| Bacillus subtilis | B15 | 39.35C |
| Pseudomonas donghuensis | Pd | 0.00D |
| Control | Control | 0.00D |
*Means with a letter in common are not significantly different (p > 0.05).

Figure 5 In vitro antagonism of the different strains of Bacillus spp., against F. oxysporum at six days of confrontation in comparison with the control. A) BIBT, B) B15, C) BITV, D) Pd, and E) Control.
For the challenge against P. aphanidermatum, the treatments were carried out 72 hours before inoculating the phytopathogen, since this oomycete showed a very fast mycelial growth by developing in the whole culture plate in only 32 hours after incubation (Fig. 6- E). Unlike the F. oxysporum assay, none of the B. subtilis strains showed inhibition against this oomycete (Fig. 6- A-C), only the P. donghuensis strain inhibited 56 % of the mycelial growth of P. aphanidermatum (Fig. 6- D) (Table 2).
Table 2 Percentage of in vitro inhibition of the different treatments against Pythium aphanidermatum at 32 hours of confrontation.
| Treatments | ||
|---|---|---|
| Strain | Code Id | Means |
| Pseudomonas donghuensis | Pd | 56.00A |
| Bacillus subtilis | BITV | 0.00B |
| Bacillus subtilis | BIBT | 0.00B |
| Bacillus subtilis | B15 | 0.00B |
| Control | Control | 0.00D |
*Means with a letter in common are not significantly different (p > 0.05).
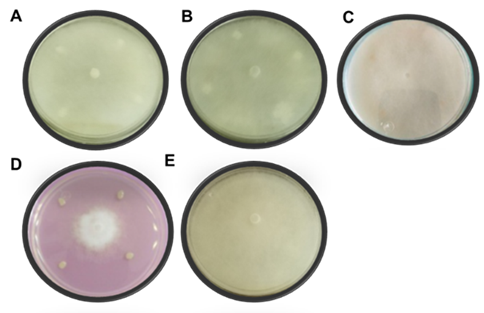
Figure 6 In vitro antagonism of the different strains against P. aphanidermatum at 32 hours after confrontation. A) BIBT, B) BITV, C) B15, D) Pd, and E) Control.
Several studies demonstrate the antagonistic capacity of the genus B. subtilis against various phytopathogens, for example, Mejía- Bautista et al. (2016) report varied inhibition ranges (21.28- 71.70 %) against two strains of Fusarium (F. equiseti and F. solani) in confrontation with 10 strains of Bacillus spp (4- B. subtilis, 1- B. cerus, 1- B. amyloliquefaciens and 4- Bacillus spp) being the strains of B. subtilis CBMT51 and CBRF8 which exhibit the highest inhibitory activity with inhibition percentages of 71.70 % and 69.92 % against F. equiseti and F. solani, respectively, while in the present study, inhibition ranges from 39.35 to 56.24 % were obtained with the three Bacillus strains against F. oxysporum. Khedher et al. (2020) reported inhibition of F. oxysporum of 54.7 % using B. subtilis, this result is similar to the present work (56.24 %) using the strain of B. subtilis (BITV). Sosa et al. (2005), confronting 17 strains of Bacillus spp., demonstrated their inhibitory capacity against several phytopathogens, being P. aphanidermatum one of them, contrasting with our research where none of said three strains of Bacillus managed to inhibit the oomycete at 32 h that lasted the experiment, unlike the work of Sosa et al. (2005) in which the P. aphanidermatum strain presented a slow growth of 72 h to fill the Petri dish. Some authors mention that the inhibition generated by Bacillus spp is due to the ability to produce some antibiotics (Iturin A and surfactin) and lytic enzymes (Asaka & Shoda, 1996; Sosa et al., 2005).
The present results obtained with P. donghuensis are similar to those proposed by Gao et al. (2015) who suggest this species is a candidate for biological control due to its high capacity for synthesis of siderophores, which is greater than other strains (Gao et al., 2012). There are reports of P. donghuensis antagonism against some phytopathogens, for example, Ossowicki et al. (2017) report growth inhibition of Rhizoctonia solani, Fusarium culmorum, Verticillium dahlia, and Pythium ultimum with P. donghuensis strain P482. This same strain (P482) was also shown to be effective against bacteria such as Dickeya solani and Pseudomonas syringae pv. syringae (Matuszewska et al., 2021). Tao et al. (2020) inhibited V. dahliae with P. donghuensis strain 22G5. Similarly, Muzio et al. (2020) reported the inhibition of Macrophomina phaseolina by P. donghuensis SVBP6. The cited authors attribute the antagonistic capacity of P. donghuensis to its high capacity for siderophore synthesis, especially of the non-fluorescent siderophore 7-hydroxytyropolone, which has not been reported in other Pseudomonas species. At present, there are still few studies carried out with this species of Pseudomonas against phytopathogenic fungi, having only reports with the strains mentioned above called P482, 22G5, and SVBP6, such is the case that at the present day, there are no results of a strain of P. donghuensis capable of inhibiting P. aphanidermatum and without inhibitory activity against F. oxysporum as observed in this work.
Conclusions
The genera F. oxysporum and P. aphanidermatum isolated from chili seedlings with symptoms of the disease and pathogenicity are confirmed as causal agents of “Damping off”.
The inhibitory effect of the antagonistic bacteria depends on the species and type of phytopathogen against which it is confronted since the isolates of B. subtilis exhibit the ability to inhibit the mycelial growth of Fusarium, but not that of Pythium, and in contrast, P. donghuensis inhibited the oomycete, but not the deuteromycete. Therefore, for future experiments, it is suggested to use mixtures of both antagonists formulated for more efficient control of “Damping off” disease.











 texto en
texto en