Introduction
Crustacean farming has grown substantially in the generation of economic wealth worldwide (FAO, 2016). In Mexico, whiteleg shrimp (Penaeus vannamei) farming is one of the most important and successful aquaculture activities. In terms of production volume, whiteleg shrimp are third place nationally however, they have the highest economic value of all the fishery resources exploited in Mexico. Similarly, shrimp is the most exported fishery resource, with the United States of America, Japan, and Italy being the main destinations. The main production area for whiteleg shrimp is northwestern Mexico (Sinaloa, Sonora, Baja California Sur, and Nayarit) (SAGARPA, 2015). As an industry, shrimp farming generates employment and has social and economic impacts. However, it also has requirements related to quality control and control of nutritional, viral, bacteriological, and parasitic diseases, reducing the negative effects on profitability (OMSA, 2008; Guzmán-Sáenz et al., 2014).
Whiteleg shrimp are the most cultivated crustacean species in Mexico and are one of the most affected by sporozoan parasites, predominantly by gregarines which are intestinal endoparasites (Prado-Garcés, 1996; Lightner, 2010). Gregarines are opportunistic parasites that have frequently caused substantial economic losses in shrimp farming (Auró & Ocampo, 2006, Morales-Covarrubias, 2010). The most common genera that infest shrimp are Nematopsis spp., Paraophioidina spp. and Cephalolubus spp. Gregarine parasitosis plays an important role in shrimp farm crops. In Latin America, it is one of the principal diseases that affect shrimp farming (Morales-Covarrubias et al., 2011; Guzmán-Sáenz et al., 2014). In shrimp of the genus Litopenaeus, large quantities of gregarines (high prevalence) are associated with empty or partially empty intestines, which coincide with a low growth rate and a possible predisposition to viral and bacterial infections and nutritional problems. (Guzmán-Sáenz et al., 2014). In some cases, massive infestations of gregarines (up to 65 % prevalence) in small-sized shrimp have produced high mortality rates (Jiménez, 1991). In Mexico, empirical methods using antibiotics are employed to control gregarines in shrimp farms yet controls and efficient application practices are lacking (Fajer-Ávila et al., 2005). Records on the effects or use of antibiotics and their efficiency are scarce.
In this high-risk industry, the growth rate of shrimp is critical to the success and profitability of farming. For this reason, our research aims to describe intestinal infestation by gregarines in whiteleg shrimp P. vannamei in farms in Nayarit, Mexico, and the use of oxibendazole Preoxol® as a parasite control method. This will inform necessary actions needed to avoid economic losses of the crop and help improve management practices.
Material and Methods
The study was carried out in the northwestern Mexico. Monitoring occurred at four farms in the state of Nayarit, Mexico, located in different areas (La Única N 21°33'42.79" W 105°16'32.65"; Franco Shrimp N 21°36'16.04" W 105°18'32.11"; Buenos Aires N 21°37'49.54" W 105°19'6.59"; Las Palmas N 21°35'44.19" W 105°18'6.70"). These farms are dedicated to cultivating whiteleg shrimp in reservoir ponds with semi-intensive production systems. Stocking density was 12 to 18 shrimp per square meter, depending on the farm and the characteristics of the ponds. Sampling was carried out during May-August; in the spring-summer cycle (dry season), and August-November; in the summer-autumn cycle (rainy season).
Sampling occurred prior to stocking post-larvae on farms. Fifty post-larvae from the supplier laboratory were sampled from each lot destined for the four farms. Once stocked, ten organisms were sampled from a pond on each farm weekly until harvest. Random sampling was used, varying the number of samples between six and eight per crop cycle for each farm. A sample of 290 shrimp per farming cycle was collected during the months of May-August and August-November, analyzing a total of 580 organisms in the four farms.
The organisms were transported to the laboratory and reviewed with the fresh analysis technique proposed by Lightner (2010), which consists of extracting the entire intestine with the help of scissors and dissection forceps. Then, each sample (intestine) was placed on a slide with 0.85 % saline solution (sodium chloride). Once the organisms were evaluated, the genera of gregarines present were identified according to Levine (1985) and Clopton (2002). Subsequently, the degree of infestation or severity was determined. For this, a count of the gregarines at different development stages found in the intestine was carried out using a manual counter (Sper brand). The classification proposed by Pantoja & Lightner (2008) was used to determine the degree of severity. Finally, the total prevalence of gregarines was estimated for the evaluated shrimp as described by Wayne (1991) using the following equation:
P= Prevalence
OI= Organisms infested with gregarines
N= Number of organisms analyzed
During sampling activities in the ponds, the salinity (PSU) was taken using a refractometer (Biomarine brand) and recorded in the field log.
Additionally, a control method against gregarines was tested in the commercial production of two shrimp farms, El Estero (N 21°56'59.69" W 105°26'58.87") and Los Otates (N 21°58'10.12" W 105 °26'36.95"), during the May-August growing cycle. For this, the antiparasitic oxibendazole with the commercial name PREOXOL® at 1.5 % was used in the feed at a rate of 2g per kg of feed in all feed rations for three days. Five 10 ha ponds were used at the El Estero farm and three 5 ha ponds at the Los Otates farm. The antibiotic was administered when the shrimp presented grade two severity or higher. Around 50 shrimp were sampled before the application of the control method and one week after the start of the treatment.
Normality (Kolmogorov-Smirnov test) and homoscedasticity (Levene's test) tests were performed on the data. Depending on the results, the non-parametric Kruskal-Wallis test was performed. A Spearman correlation was performed to determine the relationship between the presence of gregarines and salinity. All statistical analyzes were performed with the program Statistica ver. 7 from StatSoft company. The graphs were made with the SigmaPlot program ver. 12.0 from Systat Software Inc.
Results
The gregarines found in the two crop cycles belonged to the genus Nematopsis.
Phylum: Apicomplexa
Class: Sporozoa
Subclass: Gregarinia
Order: Eugregarinida
Family: Porosporidae
Genus: Nematopsis
In this genus, the sporonts form associations of two or more individuals; called syzygies, the anterior part is known as Primite, and the posterior part is known as Satellite (Figure 1a). These sporonts also form pre-reproductive associations with two or more individuals either in a chain, straight or bifurcated (Figure 1b), primite with a common epicyte slightly compressed at the junction of the deutomerite and protomerite forming a muscular neck, homogeneous protoplasm, the gametocyte presents a single nucleus, rounded protomerite, primite shorter than the length of the satellite, the nucleus generally located between the middle and posterior part of the deutomerite and satellite, also presents a gradual thinning in the last part of the syzygy.
During the sampling at the four shrimp farms, gametocytes and syzygies were generally detected in the lumen of the shrimp intestines analyzed during the two culture cycles. The trophozoites were observed, some free in the intestinal lumen and others adhered to the intestinal epithelium, the sporonts forming syzygies, generally predominating those of two associations. The epimeritus was visible in most syzygies. The gametocytes were observed in the rectal ampulla, which is in the last abdominal segment of the hindgut.
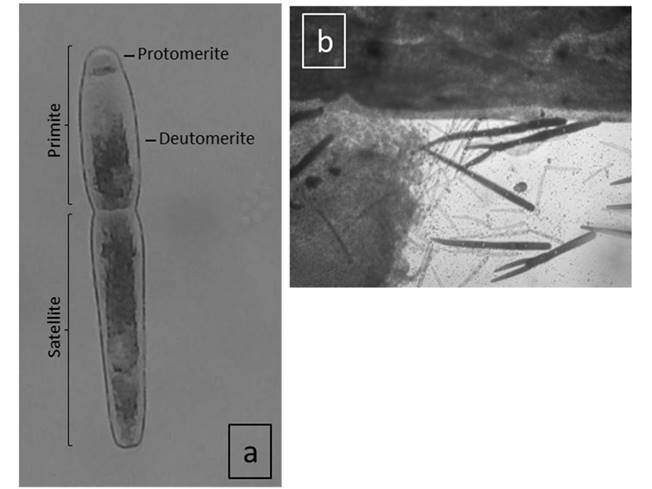
Figure 1 Morphology of gregarines. (a) Typical anatomy of sporont of Nematopsis sp (b) Bifurcated syzygy of Nematopsis sp. in the lumen of the gut.
The analyzes carried out on the 400 post-larvae from the lots that were sown in the farms showed that there was no presence of intestinal gregarines. In the samples of the organisms sown in the first (May-August) and second cycle (August-November), no presence of gregarines was observed during the first week of cultivation at any of the four monitored farms (Figure 2a and b). In the second week of cultivation, the presence of the parasite was observed in the majority of farms, except in the Las Palmas (Figure 2a) and La Única (Figure 2b) farms in the first and second cycle, respectively. From the first appearance of the parasite, it remained present until harvest (Figure 2). Regarding the mean number of gregarines per organism (Table 1), no significant differences were observed between farms during cycle 1 (p = 0.2549) nor in cycle 2 (p = 0.1517). However, significant differences were observed between both cycles (p = 0.0001).

Figure 2 Variation in the mean number of gregarines in four shrimp farms from San Blas, Nayarit. a) May-August cycle, b) August-November cycle.
Table 1 Number of gregarines (mean ± standard deviation) by farm and by crop cycle.
| Number of gregarines | ||
|---|---|---|
| Farm | May-Aug cycle | Aug-Nov cycle |
| La Unica | 106.4 ± 61.2 | 19.6 ± 18.9 |
| Franco Shrimp | 109.7 ± 63.8 | 33.0 ± 21.4 |
| Buenos Aires | 161.5 ± 88.9 | 16.0 ± 13.4 |
| Las Palmas | 98.7 ± 93.1 | 33.6 ± 17.2 |
| Mean | 119.1 | 25.5 |
In general, the prevalence of gregarines in both crop cycles was high. This ranged from 75 to 90 % in cycle 1 and 70 to 87 % in cycle 2 (Table 2). The statistical comparison did not show significant differences (p = 0.4852) between crop cycles. The degree of severity of the infestation ranged from zero to four in the first crop cycle and from zero to three in the second (Table 2). Consequently, significant differences were observed between crop cycles, being higher in cycle 1. The salinity recorded during the first crop cycle or dry period (May-August) ranged from 26 to 47 PSU, while in the rainy cycle (August-November) was from 6 to 26 PSU. During the dry season, a higher prevalence of gregarines and degree of severity were observed in the farms that presented higher salinity. However, even at lower salinities, both prevalence and degree of severity were high (Table 2). During the rainy cycle, a high prevalence of gregarines was also observed, although with a degree of severity considered low in the four farms. Only the Franco Shrimp farm which presented the highest salinity is where the highest degree of severity was recorded in this cycle (Table 2).
Table 2 Prevalence of gregarines and severity degrees in the white leg shrimp from four farms in San Blas, Nayarit.
| Farm | Prevalence (%) | Degree of Severity | Salinity (PSU) | |||
|---|---|---|---|---|---|---|
| Cycle May-Aug | Cycle Aug-Nov | Cycle May-Aug | Cycle Aug-Nov | Cycle May-Aug | Cycle Aug-Nov | |
| La Unica | 80 | 70 | Four | Zero-One | 36 ± 0.8 | 6 ± 0.9 |
| Franco Shrimp | 83 | 87 | Three-Four | Zero-Two-Three | 35 ± 0.5 | 26 ± 3.8 |
| Buenos Aires | 90 | 81 | Four | Zero-One-Two | 44 ± 2.6 | 16.4 ± 2.2 |
| Las Palmas | 75 | 85 | Two-Three-Four | Zero-Two | 27 ± 0.9 | 14 ± 1.8 |
Finally, it was observed that there was a positive correlation between the mean number of gregarines (degree of severity) and salinity (Table 3). Similarly, there was a correlation between the prevalence of gregarines and the degree of severity with respect to salinity (Table 3).
Table 3 Correlation between the prevalence of gregarines and the salinity at farms cultivating whiteleg shrimp in San Blas, Nayarit.
| Variables | Spearman R | P |
|---|---|---|
| No. of gregarines & Salinity | 0.5918 | 0.0215* |
| Prevalence & Salinity | 0.7229 | 0.0000* |
| Degree of severity & Salinity | 0.6125 | 0.0000* |
* Shows significant differences (p ≤ 0.05)
Regarding the gregarine control method, it was observed that in both farms, the treatment with PREOXOL® significantly reduced the parasite load. In general, the prevalence of gregarines after treatment decreased by more than 90 %, while the degree of severity decreased by up to three degrees (Table 4). In both farms and for all the experimental ponds, PREOXOL® was used 2 to 4 times during the crop cycle, and this allowed the endoparasite to be maintained at these prevalence levels and degree of severity until harvest.
Discussion
Gregarines are important disease-causing parasites of wild and farmed penaeid shrimp (Lightner, 1985, Lightner & Redman, 1998). Within this group, the most reported genera in shrimp are Nematopsis and Cephalolobus (Lightner, 1983). However, in the present study, only the presence of gregarines of the genus Nematopsis was found, as previously reported in eight regions dedicated to the cultivation of whiteleg shrimp P. vannamei in Latin America (including the Mexican Pacific and the Gulf of Mexico) (Morales-Covarrubias et al., 2011). In wild organisms of species such as L. setiferus, Farfantepenaeus aztecus and F. duorarum from the Gulf of Mexico, the presence of both genera of gregarines has been reported, although with a higher degree of severity (number of parasites per organism) for Nematopsis sp. (Chavez-Sanchez et al., 2002). This difference may be due to the aforementioned authors reporting the presence of the genus Cephalolobus in the stomach and of Nematopsis in the intestines, and in the present study, only the shrimps intestine were studied. However, Saavedra-Bucheli et al. (2008) report the presence of Nematopsis sp. in the stomach and hepatopancreas.
During the sampling of the post-larvae that were sown on the farms, the presence of gregarines was not observed. This parasite was not observed until the second week of culture. This indicates that the organisms were free of the endoparasite when they were stocked and contracted them in the grow-out ponds. Studies have shown that in ponds in which there is no accumulation of organic matter, as in the case of tanks covered with plastic (liner), there are fewer parasites and a higher shrimp production than in tanks with earth bottoms (Olivas-Valdez et al., 2010). The ponds used in the monitored farms have a soil bottom that allows the presence of a variety of organisms (in addition to shrimp) during cultivation, such as polychaetes, crustaceans, and mollusks. The latter group is intermediaries to complete the life cycle of gregarines (Lightner, 1983). Therefore, they could promote the presence of gregarines in the intestine of the shrimp during the second week of culture. In general, once the presence of the parasite was detected, it remained present until harvest, as has been observed in other studies (Saavedra-Bucheli et al., 2008, Calderón-Pérez, 2009, Guzmán-Sáenz et al., 2014). In this study, the mean number of gregarines per organism ranged from 25.5 to 119.1. The number of gregarines per individual can vary due to environmental conditions and even the shrimp species (Chávez-Sánchez et al., 2002, Jiménez et al., 2002, Aguado-García, 2013). For example, Guzmán-Sáenz et al. (2014) found a parasitic load in P. vanammei between 8 and 12 gregarines/organism. While Saavedra-Bucheli et al. (2008) reported mean values between 23 and 59 gregarines per intestine, and Jiménez et al. (2002), reported an oscillation between 10 and 5,000 gregarines for this same species. In farmed blue shrimp L. stylirostris, a range of 54 to 134 gregarines/organism has been reported (Saavedra-Bucheli et al., 2008).
Among the main environmental factors that have been investigated are pH, salinity, turbidity, dissolved oxygen and temperature (Gutiérrez-Salazar et al., 2011, Saavedra-Bucheli et al., 2008). Temperature has been reported to directly affect the prevalence of gregarines in whiteleg shrimp (Gutiérrez-Salazar et al., 2011), while Saavedra-Bucheli et al. (2008) report that variable salinity such as that produced during the rainy season reached an oscillation of 16 PSU, the degree of infestation by gregarines is greater. In the present study, the prevalence of gregarines did not show significant differences between cycles (cycle 1 or dry, 75-90 %; cycle 2 or rainy, 70-87 %). However, the degree of infestation was higher in the first cycle. Salinity was also different between cycles. During the dry cycle, salinity was higher (26-47 PSU), and a positive correlation between salinity and degree of severity was observed. In reference to the findings of Saavedra-Bucheli et al. (2008), the present study did not find the same pattern in the parasites behavior. Salinity variation or oscillation was 21 PSU and 20 PSU for each crop cycle, and even so, significant differences were observed in the degree of infestation between the two sampled cycles. The results of this study show that high salinities could lead to a high degree of gregarine infestation. Durán-Cobo (2016) reported a similar prevalence (49-61 %) in three salinity management schemes (10, 16, and 32 PSU) with degrees of severity of one and two. However, Olivas-Valdez et al. (2010) mention that there was no presence of gregarines in low salinity cultures (0.8-1.9 PSU).
Little information is available regarding control methods for gregarine infections. Some producers in the area report using empirical methods, such as the application of quicklime (calcium oxide) and some antibiotics, however, these are applied without proper controls or registration. In general, antibiotics for veterinary use are employed in the production of pigs, cattle, goats, and poultry. However, oxibendazole has not been used as a control method in shrimp production. This antibiotic, as with other benzamidazoles (albendazole, mebendazole, fenbendazole, triclabendazole, oxfendazole, flubendazole, and thiabendazole) have been used against monogeneans, cestodes and protozoa parasites on the skin, gills and internal organs in fish in commercial aquacultures (Athanassopoulou et al., 2009).
Due to benzamidazoles effectiveness and accepted use in fish production, our research sought to test benzamidazoles as a control treatment for gregarines in shrimp farming, with positive results. However, sulfadimethoxine and sulfamethopyrazine are among the most widely used antibiotics in veterinary medicine are due to their low cost and relatively high efficiency against parasites (MVM, 1998, Fajer-Ávila et al., 2005). As previously mentioned, there are few reliable reports on the use of pharmaceuticals to control gregarines in shrimp. However, monensin sodium and sulfachloropyridazine have been rigorously tested (Fajer-Ávila et al., 2005). These authors report that monensin sodium reduced the presence of gregarines by 92 and 94% (at a concentration of 5.5 and 6 g kg-1 of food, respectively), while sulfachloropyridazine reduced gregarines by 85 and 83% (at a concentration of 2.5 and 3.5 g Kg-1 of food, respectively), although the efficiency of both drugs can be considered high, these tests were carried out on a small scale and for a limited period. This study showed that oxibendazole at a concentration of 2g Kg-1 of food is more than 90% effective against gregarines throughout the entire production cycle.
Conclusions
In the majority of cases shrimp did not present gregarines until the second week of culture, therefore, it is important to ensure that these parasites are not present in the ponds before stocking. In addition, once the parasite was detected in the shrimp, it remained in the culture until harvest, highlighting the need for an efficient control method. Salinity directly affected the degree of severity of the gregarine infestation. We found that PREOXOL® at 1.5% was a highly efficient (more than 90%) as a control method for gregarine parasites in commercial shrimp production.











 nueva página del texto (beta)
nueva página del texto (beta)



