Introduction
Starch is the predominant storage polysaccharide of plants and is considered a natural, renewable, and inexpensive polymer, widely used in industry (Wang, S. & Copeland, 2015). This biopolymer is employed as a thickening, encapsulating, gelling and texturizing agent (Liu et al., 2017). Starch applications rely heavily on functional properties linked to granule size and shape and the structural organization of its main components (Bertolini, 2010).
There is currently a growing interest in starches with new or improved functional properties beyond those provided by starches acquired from conventional sources such as cereals and tubers (Velásquez et al., 2017; Paraginski et al., 2019). For this reason, researchers around the world have reported the functional properties and potential applications of starches obtained from unconventional sources such as the seeds of jackfruit (Artocarpus heterophyllus) (Noor et al., 2014), mango (Mangifera indica) (Tesfaye et al., 2018a), and recently, avocado fruit (Persea americana Mill.) (Rivera et al., 2019).
Avocado seeds are considered an agro-industrial by-product from the processing of avocado pulp, which generates large quantities of this residue (Tesfaye et al., 2018b). Despite this, avocado seeds hold a significant amount of starch (20-40 %), suggesting the high potential for additional re-valorization (Alves et al., 2017). Starch obtained from avocado seeds is characterized by its high apparent amylose content (32 %), its granules are triangular and circular with sizes ranging from 6-32 µm, leading to gels with opaque appearance and some level of syneresis (Chel et al., 2016; Dos Santos et al., 2016; Cornelia & Christianti, 2018; Rivera et al., 2019).
On the other hand, avocado seed starches exhibit good thickening properties, high purity, ability to form viscous pastes, and low cost (Chel et al., 2016) which are comparable to those of a conventional source such as corn (Builders et al., 2010). Often, native starches are functionally limited in applications due to their low water solubility, thermal decomposition, higher tendency to retrograde, low freeze-thaw stability, and tendency to undergo syneresis (Beninca et al., 2013). Therefore, several types of modifications are used to increase or diversify potential applications (de Oliveira et al., 2014).
Starches can be modified by physical, chemical, enzymatic, and genetic methods (Abbas et al., 2010). Acid hydrolysis (AH) is a chemical modification that alters the physicochemical properties of starch without destroying the granular structure, mainly affecting the amylose amorphous region (Hoover, 2000). AH also improves solubility, resistance to gel formation and decreases starches viscosity (Wang, Y. et al., 2003). On the other hand, among the physical modifications methods, the most widely used is heat-moisture treatment (HMT), which consists of heat treatment (>50 °C) of starch dispersions at low moisture levels (<35 %) for a certain period (15 min-24 h) (Köksel et al., 2007). HMT in starches promotes higher heat stability, crystallinity changes, increased solubility, and swelling power of granules, while viscoelastic properties are affected (Arns et al., 2014).
AH and HMT have been widely studied as starch modification methods, but the combination of AH with higher temperatures has rarely been studied. Maryam et al. (2016) evaluated the optimal conditions for obtaining dextrins from avocado seed starch by AH with HCl (0.5-0.15 N); at different temperatures (70, 80, and 90 °C) and reaction times (15, 30, and 45 min); the modification promoted a significant increase in the percentage of solubility in cold water. Therefore, the present study aimed to evaluate the effects of AH at high temperatures on the properties of regional and cv. Hass avocado seed starches. The main properties of the hydrolyzed starches were compared with their native counterparts and with a conventional source such as native and hydrolyzed corn starch, through physicochemical analysis, FTIR, XRD, SEM, and functional properties (WAI and WSI).
Material and Methods
Materials
Native seed starch from regional avocado and cv. Hass were provided by the Universidad Autónoma de Nayarit, Nayarit, Mexico. For comparative analysis, commercial corn starch was used, which was provided by the Centro de Investigación en Alimentación y Desarrollo (CIAD) Unidad Cuauhtémoc, Chihuahua, Mexico. The reagents (analytical grade) were acquired from Sigma-Aldrich (Toluca, Estado de México, Mexico).
Obtaining modified starch by AH-HMT
Native starches were modified following the methodology proposed by Maryam et al. (2016) with adaptations; Starch dispersions (30 % w/v) were prepared in 0.15 N HCl, which some were heated on a stirring hot plate (Corning PC420-D) at 70 and the others at 80 °C under constant stirring at 280 rpm for 30 min. Afterward, 100 mL of distilled water was added to each dispersion and then neutralized with sodium hydroxide (NaOH 0.05 N) to a pH of 7. The dispersions were dried in an oven at 41 °C for 72 h. Obtained solids were ground in a mortar and pestle until passing through a 150 µm sieve. Finally, samples were stored in airtight polyethylene bags until further analysis.
Physicochemical analysis, tristimulus color, apparent amylose content, and reducing sugars
Moisture (method 934.01) and ash content (method 942.05) were determined following the official guidelines of the AOAC (2002). Bulk and true density were evaluated following the methodology proposed by the Corn Refiners Association (1986). Samples tristimulus color values were measured with a Konica Minolta CR-300 colorimeter (Minolta, Osaka, Japan), and the readings were recorded in CIELAB color space (L*, a*, b*). Apparent amylose content was determined according to Williams et al. (1970) and dextrose equivalent (DE) content following the methodology of Miller (1959).
Particle Size and Scanning Electron Microscopy (SEM)
Particle morphology in the samples of native and modified starches was analyzed with an ESEM FEI QUANTA 200 scanning electron microscope (FEI Co. Eindhoven, the Netherlands) according to Gunning et al. (1999). Subsequently, the particle size distribution in six fields was analyzed using ImageJ software (ImageJ 2019, Version 1.52p).
Fourier-Transform Infrared Spectroscopy (FTIR)
Characterization of native and modified starches by FTIR was performed with an infrared spectrophotometer (Spectrum Two, PerkinElmer, Massachusetts, USA) with a UATR (Universal Attenuated Total Reflectance) accessory. Vibrational transition frequencies were recorded in wavenumbers (cm-1) within the mid-infrared. A total of 34 scans per sample were recorded with a resolution of 4 cm-1 in the region from 450 to 4,000 cm-1 (Tirado et al., 2016). Spectra were corrected by drawing a straight baseline between 1200 and 800 cm-1 and deconvolutionized with a mean width of 26 cm-1 and a deconvolution factor of 2.4 with a triangular apodization function. The absorbance intensity in the deconvoluted spectra from the baseline in the bands 1022 cm-1 and 1047 cm-1 was used to obtain the crystallinity index (1022 cm-1/1047 cm-1) (Ye et al., 2018). Finally, water affinity was determined through the ratio between 1022/995 cm-1 bands (Van Soest et al., 1995).
X-Ray Diffraction (XRD) analysis
To determine the crystalline structure of the modified and native starches, an X-ray diffractometer (Rigaku, model SmartLab), with a Cu-Kα radiation generator and high-speed D/teX detector, was used. Diffractograms were collected in a 2θ angular range from 5 to 40° with a voltage of 40 kV and a scanning speed of 4°/min.
Functional properties
The water absorption index (WAI) and water solubility index (WSI) of the starches were estimated following Anderson procedure (1969) with modifications. In brief, 0.444 g (dry basis) of the sample were weighed and placed into 15 mL polypropylene tubes (Corning ®), and 10 mL of distilled water preheated to 30 °C was added. After initiating heating, samples in suspension were placed in a water bath (30 °C, 30 min) and vortexed for 1 min at 10 and 20 min, respectively. The suspensions were centrifuged at 6,500 × g at room temperature (~24 °C), for 30 min. The volume of the supernatant (with soluble material) was measured and placed in a beaker to be dried at 100 °C for 4 h, and the tube containing the gelled insoluble material was immediately weighed.
Statistical analysis
A completely randomized design was used with a sample size of at least three replicates for each experimental analysis (n ≥ 3). The data were submitted to ANOVA and a Tukey's mean separation test (p = 0.05), using Statistical Minitab® 18.1 software (SCIENTEC, State College, Pennsylvania, USA).
Results and Discussion
Yield, physicochemical analysis, and amylose content
Yield
The recovery percentage of hydrolyzed starches with respect to their initial weight is shown in Table 1. The yield of hydrolyzed starches at 70°C ranged from 90.84 to 96.52 % without significant differences among the sources used; however, despite assessing the same HCl concentration when increasing the reaction temperature to 80 °C, the yield ranged from 89.42 to 98.14 %, observing a reduction in the yield percentage of hydrolyzed starch isolated in regional avocado (89.42 to 96.52 %). In this regard, Xia et al. (2010) reported similar behavior in hydrolyzed starches from Fritilla ussurensis and Rhizoma dioscoreae, pointing out that, based on the initial mass, hydrolysis promotes reductions in the yield of recovered starch. However, these results are more elevated than those reported by Gonçalves et al. (2014) for pine nut (Araucatia angustuifolia Bertol. Kuntze) starches (67 %) obtained by hydrolysis (22 °C, 50 days), this may be attributed to the fact that, during hydrolysis, small water-soluble molecules are released, which are discarded after centrifugation.
Moisture
Seed starches moisture content of regional avocado, cv. Hass and commercial corn, both native and hydrolyzed at 70 and 80 °C, were significantly different among the botanical sources (Table 1). The results obtained in starches hydrolyzed at 70 and 80 °C (7.03-9.23 and 7.13-7.71 %, respectively) are higher than those reported by Maryam et al. (2016), who registered moisture contents below 5.16 % in starches hydrolyzed from avocado seed, without finding significant differences between the treatments employed. According to Oelayemi et al. (2008), the moisture content in native or modified starches should be adequately low (<12 %) to avoid agglutination and proliferation of microorganisms in materials during storage.
Density
The bulk density of modified starches from avocado and corn seeds is listed in Table 1. The organization of the individual starch particles makes it a porous medium, which allows establishing two types of densities; the bulk density or apparent density determines the expansion of the starches and porosity of the products. On the other hand, true density is defined as the ratio of the mass of a sample with respect to its real volume (Bala et al., 2020). The density of hydrolyzed starches at 70 and 80 °C ranged around 0.73-0.76 g/mL, with no significant differences between treatments or botanical sources utilized. Bulk density is an assessment of thickness exhibited by samples, so it can be assumed that all samples presented smooth texture, so obtained materials could be used in the manufacture of cosmetics and as pharmaceutical excipients in tablets (Bala et al., 2020; Ashogbon, 2014; Moreton et al., 2004). On the other hand, the true density of all modified starches (0.89-0.93 g/mL at 70 °C; 0.87-0.95 g/mL at 80 °C) was higher than their respective bulk densities; these values suggest less compact materials than those reported by Manek et al. (2012) for native potato (Solanum tuberosum L.) and yellow nutsedge (Cyperus esculentus L.) starches (1.79 and 1.66 g/mL, respectively). It has been observed that free-flowing powders with coarse particles exhibit lower compaction characteristics, whereas fine powders settle quickly due to agitation (Bala et al., 2020). In general, bulk and true density are essential features to know the powders compressibility and flow properties, essential attributes in pharmaceutical formulations (Moreton et al., 2004).
Amylose content
The apparent amylose contents for hydrolyzed and native starches can be seen in Table 1. For native starches, the amylose content in hydrolyzed starches from avocado and corn seeds decreased significantly with increasing temperature during hydrolysis.
On applying a 70 °C temperature during the hydrolysis process, amylose content decreased by approximately 43, 28, and 19.8 % for regional avocado, Hass, and corn seed starches, respectively. As the reaction temperature increased to 80 °C, the apparent amylose content also declined to a concentration of 22.62, 18.08, and 9.80 % for the hydrolyzed regional avocado, cv. Hass and corn seed starches, respectively. This last one presented the highest degradation in terms of amylose content (62.24 %), followed by the hydrolyzed starch from regional avocado with degradation of 52.37 % and the cv. Hass with a loss of up to 44.78 %.
This phenomenon has extensively been reported by other authors, suggesting that the starches modification in the presence of acids such as HCl and H2SO4 leads to the cleavage of the glycosidic bonds, thus altering both the structure and properties of native starches; being the amorphous regions of the starch granule more susceptible to AH than the crystalline regions (Ahmed & Auras, 2011; Dutta et al., 2011; Miao et al., 2011).
In agreement with Wang, L. and Wang, Y. (2001), the high degradation degree of amylose was mostly caused by applying temperatures above its gelling temperature, which triggered the swelling of the granules, exposing the linear amylose chains and promoting amylose degradation. In addition, Wang, Y. and Wang, L. (2000) propose that the AH of the amorphous region of starch may be different depending on the botanical source.
Dextrose Equivalent (DE) determination
AH starch products are typified based on equivalent dextrose value, which is associated with the degree of hydrolysis. The DE values obtained for hydrolyzed starches are shown in Table 1. The DE values for hydrolyzed starches at 70 °C were 1.99, 2.14, and 2.61 for corn, regional avocado, and cv. Hass, respectively, in which no significant difference among the botanical source, was observed.
With increasing temperature, at 80 °C, the dextrose equivalent values in regional avocado and corn starches increased to 4.26 and 4.54 DE, while in modified avocado cv. Hass seed starch this increase was minimal (2.97 DE). Despite differences, the analysis of variance shows that there were no significant differences between the sources evaluated. These values are similar to those reported by Sepelevs et al. (2018), in potato starch hydrolyzed with H2SO4 (3 N at 60 °C for 3 h), who observed values of 2 DE. Additionally, Montes et al. (2008) observed values 1-5 DE for starches of yam (Dioscorea trifida) enzymatically hydrolyzed with α-amylase, which, suggests that the reaction temperature is a determining factor in the degree of hydrolysis. On the other hand, these results are lower than those previously reported by Maryam et al. (2016) on starches hydrolyzed from avocado seed (0.15 N at 90 °C for 30 min) with values of 19.6 DE.
Table 1 Physicochemical properties of native and modified avocado and corn seed starches by acid hydrolysis at 70 and 80 °C.
| Sample | Yield (%) | Moisture (%) | Ash (%) | Apparent Density (g/mL) | True Density (g/mL) | Particle Size (μm) | Apparent Amylose (%) | Dextrose Equivalent (DE) | L* | a | b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AMN | ND | 9.88 ± 0.02c | 0.10 ± 0.00a | ND | ND | 10.41 ± 0.40b | 25.96 ± 0.22c | ND | ND | ND | ND |
| ARN | ND | 9.23 ± 0.08a | 0.07 ± 0.01a | ND | ND | 18.35 ± 0.67a | 47.50 ± 1.23a | ND | ND | ND | ND |
| AHN | ND | 8.50 ± 0.19b | 0.00 ± 0.00a | ND | ND | 16.59 ± 0.60a | 32.62 ± 0.34b | ND | ND | ND | ND |
| M70 | 90.84 ± 0.28a | 7.03 ± 0.03c | 3.25 ± 0.27a | 0.74 ± 0.01a | 0.92 ± 0.00ab | 20.31 ± 1.41b | 20.83 ± 0.37c | 1.99 ± 0.24a | 95.51 ± 0.05a | -1.31 ± 0.02c | 5.42 ± 0.07b |
| AR70 | 94.46 ± 1.67a | 9.23 ± 0.08a | 2.76 ± 0.11a | 0.73 ± 0.00a | 0.89 ± 0.01b | 23.00 ± 1.61a | 27.07 ± 0.08a | 2.15 ± 0.32a | 79.93 ± 76b | 5.21 ± 0.10a | 11.05 ± 0.24a |
| AH70 | 96.52 ± 2.56a | 8.50 ± 0.19b | 3.08 ± 0.09a | 0.74 ± 0.01a | 0.93 ± 0.01a | 25.29 ± 2.37a | 23.48 ± 0.17b | 2.61 ± 0.03a | 80.77 ± 0.10b | 4.57 ± 0.05b | 11.37 ± 0.07a |
| M80 | 98.14 ± 0.35a | 7.13 ± 0.03b | 2.53 ± 0.16b | 0.75 ± 0.01a | 0.87 ± 0.01b | 15.31 ± 1.57a | 9.80 ± 0.08c | 4.54 ± 0.04a | 92.25 ± 0.06a | -0.25 ± 0.01c | 7.84 ± 0.13c |
| AR80 | 89.42 ± 1.78b | 7.71 ± 0.15a | 2.94 ± 0.05ab | 0.74 ± 0.00a | 0.94 ± 0.00a | 16.63 ± 1.10a | 22.62 ± 0.22a | 4.26 ± 0.62a | 82.35 ± 0.30b | 2.86 ± 0.03b | 9.63±0.04b |
| AH80 | 96.20 ± 0.10a | 7.35 ± 0.14ab | 3.27 ± 0.09a | 0.76 ± 0.01a | 0.95 ± 0.01a | 18.57 ± 1.46a | 18.01 ± 0.30b | 2.97 ± 0.13a | 82.14 ± 0.14b | 4.52 ± 0.04a | 11.28 ± 0.06a |
*Different letters within each column are significantly different p <0.05. MN: native corn starch; ARN: starch from regional avocado seeds; AHN: starch from avocado cv. Hass seeds. M70, AR70, AH70: starches from corn, regional and cv. Hass avocado seeds, hydrolyzed with 0.15 N HCl at 70 °C. M80, AR80, AH80: corn, regional and cv. Hass avocado seeds starches, hydrolyzed with 0.15 N HCl at 80 °C.
Colorimetry
Color analysis of the samples highlighted significant differences among the parameters evaluated (Table 1). Hydrolyzed starches at 70 °C showed a higher lightness (L*), suggesting whiter starches. Values of 79.93 and 80.77 were recorded for avocado starches (regional and cv. Hass, respectively); while the highest value (L*= 95.51) was recorded for corn hydrolyzed starch at this temperature. Despite the increase in reacting temperature (80 °C), no significant changes were recorded in starch whiteness. Similar results have been documented by Gonçalves et al. (2014) for hydrolyzed pine nut starches, with L* values of 94.98.
Starch hydrolyzed from avocado seeds at 70 °C, showed positive values in a (4.57-5.21) and b (11.05-11.37) exhibiting pinkish hues, a feature describable of starches extracted from the avocado seed, as a result of seed oxidation (Maryam et al., 2016). In hydrolyzed avocado starches at 80 °C, a decreased (2.86 - 4.52) as an effect of the treatment used; even, *L values increased from 79-80 to 82 in avocado seed starches. On the other hand, the modified corn starches showed a decrease in their whiteness value from 95 to 92; despite this trend, these values are higher than those recorded for avocado seed starches.
Particle size and morphology
SEM analysis evidenced changes in size (Table 1) and shape (Figure 1) of starch granules post hydrolysis process, regardless of the botanical source. The native starch granules of avocado seeds presented spherical and oval shapes, with soft and smooth surfaces, with average sizes of 16.59 and 18.35 µm in cv. Hass and regional varieties, respectively. These results agree with what has been reported for starches in several avocado cultivars (Builders et al., 2010; Chel et al., 2016; Alves et al., 2017) (Figure 1d, g). On the other hand, in native corn starch granules, they present polyhedral shapes, with average sizes of 10.41 µm, which is below that reported by Bustillos et al. (2018) for native corn starches (13.3 - 21.4 µm).
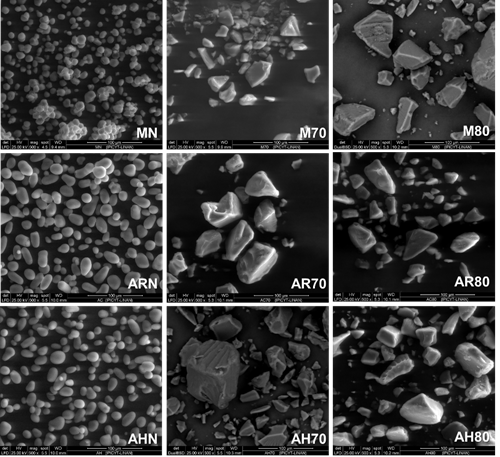
MN: native corn starch; ARN: starch from regional avocado seeds; AHN: starch from avocado cv. Hass seeds. M70, AR70, AH70: starches from corn, regional avocado seeds and cv.Hass avocado, hydrolyzed with 0.15 N HCl at 70 °C. M80, AR80, AH80: corn starches, regional avocado seeds and cv. Hass, hydrolyzed with 0.15 N HCl at 80 °C.
Figure 1 Scanning electron microscopy micrographs of native and modified starches by acid hydrolysis at 70 and 80 °C, from avocado and corn seeds.
For native starch granules (Figure 1 a, d, g), micrographs showed that modification by AH at 70 (Figure 1 b, e, h) and 80 °C (Figure 1 c, f, i) resulted in the loss of granular structure with the presence of larger particles with irregular shapes. The loss of the granular structure of the starch granules was attributed to gelatinization of the samples in the presence of HCl during the hydrolysis process, which resulted in the formation of irregular crystal structures (Figure 1b, c, e, f, h, i) (Putaux et al., 2003). Similar results have been reported for pre-gelatinized wheat (Triticum aestivum L.) starches (Majzoobi et al., 2011).
The micrographs in Figure 1 show that the hydrolyzed avocado seed starches exhibited larger particle sizes (~23 µm) at 70 °C; which decreased as hydrolysis temperature increased to 80 °C (16.63 - 18.57 µm, cv. Hass and regional, respectively). Similar patterns were observed for the modified corn starches, whose particle sizes were 20.31 µm and 15.31 µm at 70 and 80 °C, respectively.
Particle size differences in the hydrolyzed starches from avocado and corn seeds evaluated in this study can be derived from the amylose content of the granules in their native form and the XDR pattern. According to Kim et al. (2012), starches with high amylose content with type B crystallinity generate larger particle size after performing AH modification on granules, which is mainly attributed to a higher resistance to hydrolysis.
Fourier-Transform Infrared Spectroscopy (FTIR)
Figure 2 shows the deconvoluted FTIR spectra of native and modified starches. The FTIR spectra of native starches reveal three main bands at 1047, 1022, and 995 cm-1. The bands at 1047 and 1022 cm-1 correlate to the crystalline (amylopectin) and amorphous (amylose) zones, respectively (Sevenou et al., 2002); while the band at 995 cm-1 is mainly associated with the water-associating capacity (Xia et al., 2010).
After the modification process, no significant change in the intensity of the 1047 cm-1 band was observed in the modified avocado seed starches; however, it was noted that this band presented a reduction in the hydrolyzed corn starches, thus supporting the theory of Putaux et al. (2003), which suggests that corn starches are more susceptible to be hydrolyzed, given that the acid initially penetrates inside the branched amylopectin molecules, being a characteristic of cereal starches that present an A-type XRD pattern. On the other hand, the band at 1022 cm-1, presented a decrease in its relative intensity in all the evaluated sources, this performance has been attributed to the high susceptibility of linear molecules with α (1-4) bond to the AH process (Khatoon et al., 2009). As the hydrolysis temperature increased, the intensity of the 1022 cm-1 band decreased and an increase of the band at 995 cm-1 is distinguished, this is a consequence of the loss of ordered structures inside the granule (Sevenou et al., 2002). According to van Soest et al. (1995) and Xia et al. (2010), the variations in the intensity of the band at 995 cm-1 are linked to the bending vibrations of the COH bonds, which are sensitive to water content. On the other hand, the 1022/995 cm-1 ratio has been used as an indicator of the characteristics of more hydrophilic materials (Sevenou et al., 2002) (Table 2).
Native corn starch (0.80) had a higher degree of water affinity compared to starches from cv. Hass and regional avocado seeds (0.75 and 0.77, respectively). These results are lower than those described by Sevenou et al. (2002) for native corn starch (0.98) but higher than potato starches (0.40) and high amylose corn (0.53). After modification by AH with hydrochloric acid, an increase in the ratio 1022/995 cm-1 for all botanical sources, was observed. When the hydrolysis temperature was 70 °C, corn starch (0.86) presented a higher affinity for water compared to regional (0.84) and cv. Hass (0.83) avocado seed starches. In this context, avocado starches exhibit a higher level of organization in their external region, which delays hydrolysis as opposed to corn starch (Sevenou et al., 2002). Finally, when the temperature during the hydrolysis process increased from 70 to 80 °C, the affinity for water did not show significant differences.
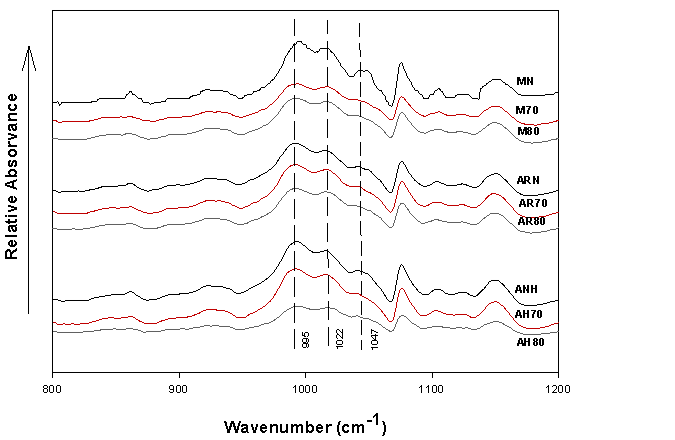
Figure 2 Deconvoluted FTIR spectra of native and modified avocado and corn seed starches by acid hydrolysis at 70 and 80 °C.
The ratios of the absorbance intensity of the 1047/1022 cm-1 bands are shown in Table 2. This ratio indicates the level of molecular order on granule surface (crystallinity index) (Aboubakar et al., 2008). Among the native starches, corn starch presented the highest crystallinity index (0.63), followed by starches obtained from cv. Hass and regional avocado seeds (0.61 and 0.59, respectively); this characteristic is closely related to XRD patterns (Sevenou et al., 2002). After modification, a decrease in the crystallinity index is perceived, suggesting that the ordered structure of native starches was broken due to amylose degradation by HCl (Hoover, 2000).
Table 2 IR ratio of absorbance 1047/1020 cm-1, 1022/995 cm-1, and crystallinity percentage of native and modified avocado and corn seed starches by acid hydrolysis at 70 and 80 °C.
| Sample | Ratio 1047/1022 cm-1 (Crystallinity index) | Ratio 1022/995 cm-1 (Water affinity) | Crystallinity (%) |
|---|---|---|---|
| MN | 0.63 ± 0.01a | 0.80 ± 0.01a | 27.85 ± 0.36a |
| ARN | 0.59 ± 0.00b | 0.77 ± 0.00b | 23.92 ± 0.54b |
| AHN | 0.61 ± 0.00ab | 0.75 ± 0.00b | 26.22 ± 0.61a |
| M70 | 0.59 ± 0.00a | 0.86 ± 0.01a | 24.28 ± 0.84a |
| AR70 | 0.56 ± 0.01b | 0.84 ± 0.01ab | 25.91 ± 0.34a |
| AH70 | 0.57 ± 0.00ab | 0.83 ± 0.00b | 24.54 ± 1.08a |
| M80 | 0.58 ± 0.00a | 0.86 ± 0.01a | 24.79 ± 0.68a |
| AR80 | 0.56 ± 0.00a | 0.53 ± 0.01b | 28.80 ± 1.50a |
| AH80 | 0.58 ± 0.01a | 0.86 ± 0.01a | 30.20 ± 2.39a |
*Different letters within each column are significantly different p <0.05. MN: native corn starch; ARN: starch from regional avocado seeds; AHN: starch from cv. Hass avocado seeds. M70, AR70, AH70: starches from corn, regional avocado seeds and cv. Hass avocado, hydrolyzed with 0.15 N HCl at 70 °C. M80, AR80, AH80: corn starches, regional avocado seeds and cv. Hass, hydrolyzed with 0.15 N HCl at 80 °C.
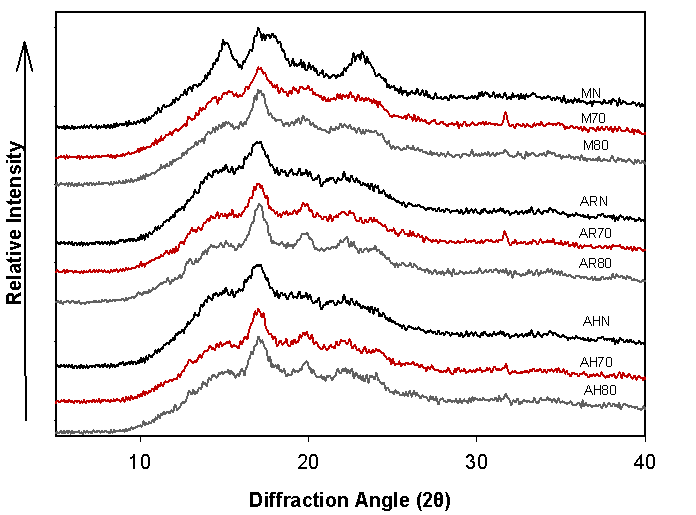
X-ray Diffraction (XRD)
XRD patterns of native and hydrolyzed starches from avocado (regional and cv. Hass) and corn seeds are presented in Figure 3. In general, the XRD patterns of native and hydrolyzed starches from regional avocado and cv. Hass, exhibit a B-type crystallinity pattern, with typical peaks at 15°, 17°, 19°, and 22° at the 2θ angle. Similar results to those reported by Kahn (1987) for native avocado and cv. Zutano seed starches (15°, 17°, 19° and 22° 2θ). No changes were observed in the diffraction patterns of native and hydrolyzed avocado starches; however, an increase in the crystallinity peaks at 17°, 22°, and 24° 2θ was observed in the AH-modified starches of both avocado varieties. This condition can be a consequence of the temperature used during the modification process, since the higher the temperature, the higher the degree of hydrolysis (Montes et al., 2008).
On the other hand, the XRD pattern of native corn starch exhibits an A-type pattern, typical of cereal starches (15 °, 17°,18° and 23° 2θ) (Utrilla et al., 2014). These peaks, decreased in intensity during the AH process, observing a decrease as reaction temperature raised. Consequently, the XRD pattern of corn changed to type B with peaks at 15°, 17°, 19°, 22° and 31° (2θ) in hydrolyzed starches at 70 and 80 °C. Although the acid attacks mainly the amorphous regions of the granule, this may be different relying on the botanical source, in terms of dimension and molecular arrangement (Wang, L. & Wang, Y., 2001). In comparison with avocado seed starches, a greater affectation caused by acid on corn starch is evident, despite its low amylose content (25.96 %), so that the acid may affect the amylopectin (α 1-4) long chains, causing a crystallinity decrement of hydrolyzed corn starches (Biliaderis et al., 1981).
Table 2 shows the percent crystallinity for native and modified starches obtained from X-ray diffractograms. Usually, the percent crystallinity correlates negatively with amylose content (Biliaderis et al., 1981). In the case of native starches, the percentage crystallinity varied significantly in the following decreasing order: MN>AHN>ARN.
In general, the percentage of crystallinity tends to increase after the hydrolysis process as a function of temperature and acid exposure time as a result of the degradation of the amorphous region (Man et al., 2012; Montes et al., 2008; Wang, L. & Wang, Y., 2001). When avocado starch was hydrolyzed at 70 °C, a gradual increase in crystallinity from 23.92 to 28.80 % and from 26.22 to 30.20 % was observed in regional and cv. Hass avocado, respectively. This trend has been widely reported for diverse botanical sources such as jackfruit and lotus (Nelumbo nucifera Gaertn), which exhibit B-type diffraction patterns (Dutta et al., 2011; Man et al., 2012). Furthermore, these results are in agreement with the behavior of amylose content reported in Table 1, where a decrease in amylose content (~50 %) was observed as a result of the hydrolysis process. This is attributed to the preferential hydrolysis of the amorphous domains by the acid (Xia et al., 2010).
On the other hand, in an inverse pattern, the percentage of crystallinity of native corn starch decreases when hydrolyzed at 70 °C, and remains unchanged as the reaction temperature increases to 80 °C (27.85>24.28>24.70 % of crystallinity, respectively). This behavior in corn starches occurs as a consequence of the ring depletion in the amorphous region, the acid can act on the crystalline structures in the interior of the granule. This behavior is reflected in the X-ray diffraction patterns for starch, where it changes from type A to B after the hydrolysis process (Utrilla et al., 2014).
Functional properties
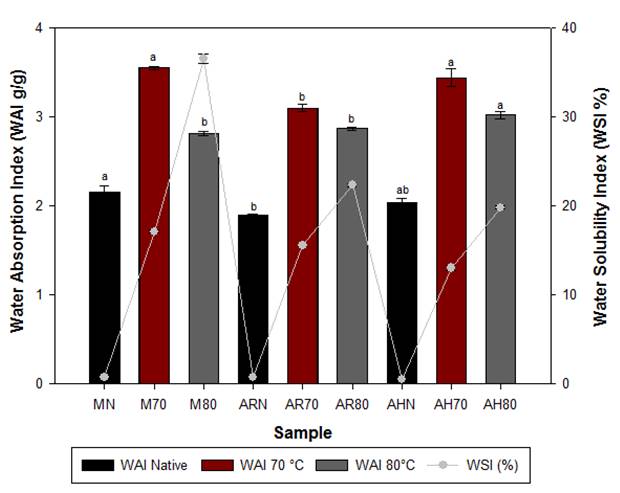
The water absorption index (WAI) and water solubility index (WSI) are dependent on several factors, such as botanical source, amylose/amylopectin ratio, extraction method, and thermic history of the samples (Singh & Smith, 1997). Hydrolyzed starches from all sources used in comparison with their native counterparts showed an increase in WAI and WSI values, which is largely attributed to macromolecular disorganization and degradation of starch granules during exposure to hydrochloric acid and heat treatment (Nakorn et al., 2009).
For hydrolyzed starches at 70 °C, WAI changed significantly in the following decreasing order: M70>AH70>AR70; similar relationships have been reported for hydrolyzed corn and pregelatinized high amylose rice starches (Köksel et al., 2007; Nakor et al., 2009).
In Figure 4, it is unmistakable that increasing the temperature during hydrolysis promoted further breakdown of the granular structure and, therefore, a decrease in the WAI of hydrolyzed starches at 80 °C was observed in starches from the three botanical sources (AH80>AR80>M80). This effect was followed by an increase in the soluble component released from the broken granules, which led to an accelerated increase in the solubility percentage of the hydrolyzed starches, showing significant differences between starches from avocado and corn seeds.
Conclusions
The physicochemical, structural, and functional properties of avocado and corn seed starches gradually altered with increasing temperature, which is considered a significant factor in the hydrolysis process. After modification, the starch granules of the three botanical sources lost granular structure resulting in the formation of larger particles (agglomeration) and small polyhedral particles. The loss of granular structure due to AH at high temperatures promoted further degradation of amylose by acid and increased leaching of soluble components, resulting in increased starch crystallinity and functional properties (WAI and WSI) at low temperatures. However, although hydronium ions attack mainly the amorphous regions of starch granules, in the case of corn starch, more damage was observed due to the breaking of the α 1-4 bonds of the amylopectin chains; substantially modifying its XRD pattern. Hydrolyzed starches from avocado seeds could be used in the non-food industry, as adhesive raw materials, paper industries, textiles, construction materials, or as a solvent mixture of ingredients in insecticides and fungicides.











 nueva página del texto (beta)
nueva página del texto (beta)