Introduction
Monensin sodium is a component produced by Streptomyces cinnamonensis species. In cattle, it is used to increase feed efficiency and milk production, said benefits are due to alterations in ruminal microflora; control of coccidia; improved efficiency of bacteria involved in the production of energy and fats in milk; decreased ketosis; prevention of acidosis and pulmonary problems (Novilla, 2018; Pereira et al., 2019; Roder, 2011), in addition, monesin sodium reduces feed consumption and methane production without affecting the performance of cattle (Tedeschi et al., 2003).
The use of monesin sodium in therapeutic doses does not cause adverse effects. However, a common accidental intoxication may occur by mixing ingredients, ingesting premixes or altered concentrated products, incidental consumption by non-target species or by ingestion of poultry waste (Roder, 2011), and even potentiate intoxications by other metals such as copper (Lopes et al., 2022).
In animal cells, monesin sodium acts as an ionophore that moves monovalent cations such as calcium and sodium through the cell wall in exchange for potassium and hydrogen ions (Ensley, 2020; Łowicki & Huczyński, 2013). Binding to these ions can cause loss of intracellular potassium, resulting in inhibition of ATP production in mitochondria, and as a result, energy production is decreased leading to cell death. This cell death causes damage to several tissues, including the heart, which in turn affects other organs (Aleman et al., 2007; Zachary, 2022).
Several intoxications have been reported in productive species such as cattle (Andrade et al., 2020; Omidi et al., 2008), buffalo (Rozza et al., 2006), goats (Deljou et al., 2014), sheep (Ashrafihelan et al., 2014) pigs and poultry (de Carvalho et al., 2021; Roder, 2011). However, in Mexico, in intensive dairy production systems, this phenomenon has not been described. This work aimed to report a case of accidental monensin sodium poisoning in an intensive dairy production system in northern Mexico.
Materials and methods
In this report, a case referred to the Laboratory of Clinical Analysis and Veterinary Pathology from La Laguna S.C. in collaboration with the laboratory of the Veterinary Diagnostic Unit from the Universidad Autónoma Agraria Antonio Narro Unidad Laguna, is described. The poisoning events occurred in August 2021 in a dairy herd of La Comarca Lagunera in northern Mexico. 306 five-month-old female Holstein-Friesian animals from the same farm were accidentally fed with a ration containing a high concentration of monensin sodium. The ration consisted of a mixture of alfalfa hay, a commercial balanced solid feed mixed with an erroneous amount of monensin sodium of 16 mg/kg which was included to improve feed conversion and as a coccidiostat.
The affected animals presented different signs such as diarrhea, depression, anorexia, ataxia, dyspnea, sialorrhea, tachycardia, jugular distension, ruminal atony, edema, and dehydration. Blood samples were taken for serum creatine phosphokinase (CPK) measurement on an automated clinical chemistry analyzer (H-100, HLAB®). Among dead animals, nine were necropsied and samples were taken for histopathological evaluation including heart, lung, and liver.
Once the poisoning with monesin sodium was diagnosed, the diet was suspended and since there is no antidote for such type of poisoning (Gonzalez et al., 2005), vitamin E and selenium were administered as antioxidants and membrane protectors, in addition to a B-complex-based liver protector. The diet was changed and the number of deaths decreased to zero.
Results and discussion
The addition of ionophores such as monesin sodium in the cattle diet is a common practice. Due to its multiple benefits, it has become an indispensable element in cattle farms (Novilla, 2018). However, when an elevated concentration occurs, not only prokaryotic cells are affected but also the eukaryotic cells of the animal, resulting in severe cellular damage. Unlike equines (Aleman et al., 2007), cattle are less susceptible to the adverse effects of ionophores. But this margin of safety is reduced if the concentration is increased (Ensley, 2020). As far as we are aware, the present study is the first report from northern Mexico of monensin sodium poisoning in Holstein-Friesian cattle.
In the present work, from the 306 animals that were exposed to monensin sodium, 82 died within 30 days (26.79 % mortality). The dose offered was higher than the recommended oral dose (1 mg/kg). In cattle, the LD50 is 50 - 80 mg/Kg (Zachary, 2022) although some authors consider the 50-50 lethality from 26 mg/Kg (Łowicki & Huczyński, 2013). Based on the dose offered and the mortality percentage, these results agree with the lethality reported by Łowicki & Huczyński (2013). The percentage of mortality depends on the ingested dose; up to 100 % have been reported with very high doses (22 388 mg/kg) (Ashrafihelan et al., 2014). Similarly, the signs that manifest are largely dose-dependent. Although, whenever mortality is reported most of the signs coincide. In the present work, signs such as diarrhea, depression, anorexia, ataxia, dyspnea, sialorrhea, tachycardia, ruminal atony, edema, and dehydration were observed. This matches with other intoxication studies (Andrade et al., 2020; Ashrafihelan et al., 2014; Garcia et al., 2020; Omidi et al., 2008). Most of the above-mentioned signs are a consequence of the alteration that takes place in tissues with high energetic activity such as muscle, mainly cardiac and skeletal, as well as nervous tissue causing mitochondrial failure and depletion of cellular adenosine triphosphate (ATP) (Ashrafihelan et al., 2014; Litwak et al., 2005; Omidi et al., 2008).
In the present case, a biochemical analysis is of great help to determine cell damage and its monitoring. Here, the mean biochemical analysis of CPK was 2913.6 ± 2402.29 (SD). These results vary depending on the severity of the injury. When CPK levels are 1639.8 ± 2797.31 (SD) less mortality has been reported as opposed to large concentrations, such as 30115 ± 1703 (SD), where mortality has been higher (Andrade et al., 2020). This biomarker, like cardiac troponin I, is an important support to indicate cardiac necrosis or severity of myocardial damage that can be used to assess progression or recovery from muscle damage (Andrade et al., 2020; Gonzalez et al., 2005; Nogueira et al., 2009).
In the necropsies performed, the main macroscopic findings consisted of degenerative myopathy with multifocal whitish areas in the heart (Figure 1), hydrothorax (Figure 2), hydropericardium, congestion, and pulmonary edema, these alterations were reported as a consequence of heart failure. Similar lesions have been found in other reports of ionophore poisoning (Andrade et al., 2020; Bence et al., 2018; De La Cruz-Hernandez et al., 2012; Rozza et al., 2006). From the most important organs, samples were taken for histological evaluation, which revealed that the main lesions were found in the heart, lung, and liver. Of the necropsied calves, 100% showed suppurative or non-suppurative myocarditis with degeneration of myocardial fibers (Figure 3). Suppurative and necrotic hepatitis was found in the liver. 100% presented some type of fibrinous, suppurative, or fibrinopurulent bronchopneumonia with fibrinous pleuritis and necrotic vasculitis. 55.55 % showed fibrinoid arteritis and 55.55 % pulmonary congestion and edema. Cardiac tissue is one of the most affected organs due to its high kinetic activity, and the liver is affected due to congestion and to the fact that ionophore metabolism takes place in this organ. Heart and liver alterations have been previously reported with similar lesions (Andrade et al., 2020; Bence et al., 2018; De La Cruz-Hernández et al., 2012; Omidi et al., 2008). However, lung damage is atypical in this type of poisoning. The greatest possibility is that it is an isolated event since these lesions have not been reported previously. The absence of previous respiratory infections in the exposed animals and the finding of pulmonary alterations in all the calves studied suggests that it could be related to the poisoning, probably due to systemic inflammatory response syndrome (SIRS). However, given the absence of a control group, we cannot fully conclude with this finding. It is suggested that emphasis should be placed on lung tissue damage for future reports.
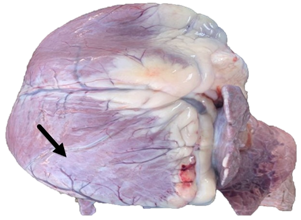
Figure 1 Degenerative myopathy with muscle pallor (arrow) is observed in a calf heart intoxicated with monensin sodium.
Conclusion
Based on the clinical, biochemical, morphological, and histopathological findings, it is strongly suggested that this is a case of monensin sodium intoxication in calves. It is important to note that since its discovery, many cases of toxicosis have been, and continue to be reported, which shows how easy it is to incur by human mistakes that lead to poisoning. It is recommended to breeders and related people be very careful when handling these substances.











 nueva página del texto (beta)
nueva página del texto (beta)





