Introduction
The main goal of any cow-calf production system (beef production) is to obtain one calf per cow per year, because this is the factor with the greatest economic impact in any cattle production unit (Hess et al., 2005; Mejía-Bautista et al., 2010). Thus, estrus manifestation within a relatively short period after calving (≤90 days) is of paramount importance, since it optimizes the reproductive performance of cows and guarantees a steady calf production (Hess et al., 2005; Martins et al., 2012). Despite the productive and economic importance of postpartum anestrus in cattle (Ciccioli et al., 2003; Mejía-Bautista et al., 2010), the reproductive performance of beef cows in tropical regions is poor, leading to parturition-to-conception intervals ≥300 days and conception rates at 45-55 % (Mejía-Bautista et al., 2010; Diskin & Kenny, 2016). These conditions have been attributed mainly to the effect of the cow-calf bond during the suckling period (Galina et al., 2001; Crowe et al., 2014). In addition to the inhibitory effect of the calf presence on the resumption of the postpartum ovarian activity in the cow, if the dam does not have an appropriate nutritional management and/or good body condition (BC), the postpartum ovarian inactivity will remain, prolonging the interval calving-to-conception (days open) (Crowe, 2008; Diskin & Kenny, 2014; Bayemi et al., 2015; Stevenson et al., 2015; Diskin & Kenny, 2016).
Therefore, it becomes a priority to control the interaction suckling-cow nutrition, as it allows an early resumption of postpartum ovarian activity and early conception (≤90 days postpartum) (Wettemann et al., 2003; Quintans et al., 2009; Watanabe et al., 2013; Crowe et al., 2014; Diskin & Kenny, 2016). Early weaning and restricted or controlled suckling are the management tools most commonly used to shorten the anestrus postpartum period and therefore to increase the reproductive efficiency of cattle (Galina et al., 2001; Montiel & Ahuja, 2005; Waterman et al., 2012; Barreiros et al., 2014).
In cows, feed supplementation during the postpartum period contributes to reduce the anestrus length, increasing their probability to become pregnant sooner after calving (Wettemann et al., 2003; Watanabe et al., 2013). Thus, cows that kept on a good plane of nutrition during the postpartum period will have good BC and better reproductive performance (Stagg et al., 1998; Crowe, 2008; Stevenson et al., 2015). Nonetheless, the use of this management tool in tropical regions is limited (Galina et al., 2001; Mejía-Bautista et al., 2010) due to insufficient information on how to use it (Galina et al., 2001; Vendramini et al., 2006; Mejía-Bautista et al., 2010; Hernández-Martínez et al., 2011), on the cost of feed supplementation compared to traditional weaning (7-8 months), and the constant handling of the animals that may be required (Galina et al., 2001; Blanco et al., 2009).
Nevertheless, early weaning, restricted suckling and feed supplementation of cows are practical and cost-effective management tools for cow-calf production systems, compared to traditional management (no early weaning, no feed supplementation of the cow); moreover, early weaning improves BC and weight gain in grazing cows, which is necessary to obtain good results during the breeding season (Galina et al., 2001; Quintans et al., 2004; Blanco et al., 2009; Waterman et al., 2012; Martins et al., 2012).
Thus, along with early weaning, it is necessary to offer feed supplementation to cows in order to diminish or inhibit the negative effects caused by the nutritional status and suckling on the postpartum follicular development and ovulation, which affect the parturitionto-conception interval. Therefore, the objective of the study was to determine the effect of restricted suckling and feed supplementation on the postpartum follicular development and serum concentrations of progesterone and estradiol in Simbrah cows.
Material and Methods
All the procedures conducted during the study were in compliance with the Official Mexican Standard NOM-062-ZOO-1999 on the Technical specifications for the production, care and use of laboratory and experimental animals.
Study location
The study was conducted at the cattle ranch San José, which is devoted to the production of beef calves. The ranch is located in Macuspana, Tabasco, Mexico, at 17° 45′ 17″ north latitude and 92° 33′ 32″ west length, at 10 masl, with tropical climate, mean annual temperature 26.4 °C and annual precipitation 3,186 mm (INEGI, 2018).
Characteristics of experimental animals
A total of 120 early postpartum (1-5 days), lactating and multiparous (2-5 calvings) Simbrah cows were selected. Body condition score of the cows ranged from 2 to 4 in a scale of 5 points (Pullan, 1978), and body weight averaged 468.5 ± 13.2 kg. The cows were treated against internal parasites (Levamisol, Lab. Genfar, 1 mL/20 kg live weight via IM) and external parasites (Bayticol® Pour-on 1 %, Lab. Bayer, 10 mL/100 kg live weight), were given vitamins (Vigantol® ADE, Lab. Bayer, 5 mL via IM), and were vaccinated against rabies (Nobivac® Rabia, Lab. MSD), anthrax (vacuna anticarbonosa, Lab. MSD) and blackleg (Bacterina triple C.E.S.®, Lab. MSD).
Experimental design
Cows were randomly distributed in a 2 x 2 factorial design. On Day 0 (zero) of the study, cows were assigned to one of the following treatments: T1) Continuous suckling with feed supplementation (CCC; n=30), T2) Restricted suckling without feed supplementation (RSC; n=30), T3) Restricted suckling with feed supplementation (RCC, n=30) and T4) Continuous suckling without feed supplementation (CSC, n=30). All the cows were managed under rotational grazing in 150 ha divided in 30 paddocks of 5 ha each, sown with MG5 (Brachiaria brizantha, 12 % crude protein [CP]) and humidicola (Brachiaria humidicola, 7.5 % CP) grass, and all cows received ad libitum a supplemental source of salt and minerals (8 % phosphorus) from their inclusion in the study (Day 0) until diagnosed as pregnant. Cows from treatments T1 and T3 received additionally a commercial feed supplement (19 % CP and 73.26 % TDN) at 4 kg/cow/ day from Day 0 until ovulation Day. Cows belonging to each treatment group were kept together and separated from those from the other groups.
Suckling type
Continuous suckling (T1 and T4) consisted on the calf staying with its mother since birth until weaning, at 210 days old. Restricted suckling (T2 and T3) consisted on the calf staying with its mother since birth until 15 days old; then, the calf was separated from its mother and was allowed to suckle once a day in the morning (0700 to 0800 h) until weaning, at 105 days old.
Study variables
Follicular dynamics and ovulation
To determine the follicular dynamics, the ovarian structures were examined through transrectal ultrasonography (US, portable ultrasound Mindray model DP-10 VET, with 7.0 MHz transrectal transducer) twice a week since Day 0 (start of the study) until detection of ovulation, which was considered as indicative of resumption of estrus cyclicity. To characterize the changes in the ovarian structures, in each US evaluation all the follicles present were measured, and the presence and size of a dominant follicle (DF) were determined. In the cows that had a 12-15 mm diameter DF that had disappeared in the following US evaluation, then further US evaluations were conducted on Days 7 and 14 after the DF disappearance to confirm ovulation through the detection of a corpus luteum (CL). If a CL was not found, US evaluations were resumed and conducted twice a week until finding one. To monitor the follicular dynamics, the transrectal transducer from the US apparatus was inserted through the rectum and placed along the dorsal surface of each uterine horn, moving it sidewise to examine the ovaries; the reproductive tract was not directly manipulated before or during the US evaluation (Ginther et al., 1989; Taylor et al., 1993).
Blood sampling and determination of serum concentrations of progesterone and estradiol 17-β (E2)
In all cows, serum progesterone (P4) concentrations were determined to confirm the functionality of the CL (≥1 ng/mL P4 in two consecutive samplings; Rodríguez-Sánchez et al., 2015), and serum estradiol 17-β (E2) concentrations were measured as indicative of follicular activity. Blood samplings were conducted on the same days as the US evaluations. Blood was drawn from the coccygeal vein with 21 G x 38 mm sterile needles into 6 mL Vacutainer® tubes with no anticoagulant. Blood samples were centrifuged at 2500 rpm for 10 min within 4 hours after collection to obtain the serum, which was separated into aliquots and stored at -20 °C until hormone determination. Concentrations of P4 (ng/mL) and E2 (pg/ mL) were measured through solid phase enzyme-linked immunosorbent assay (ELISA) using commercial kits (Progesterone EIA-1561 and Estradiol EIA-2693, DRG Diagnostics, Germany). The progesterone assay showed cross-reactivity with progesterone (100 %); the range of the curve was 0.30-40 ng/mL, the assay sensitivity was 0.40 ng/mL and the inter assay coefficient of variation was 6.63 %. The estradiol assay showed cross-reactivity with estradiol-17β (100 %), estrone (6.86 %) and estriol (2.27 %); the range of the curve was 25-2000 pg/mL, the assay sensitivity was 50 pg/mL and the inter-assay coefficient of variation was 7.78 %. The ELISA microplates were read at 450 nm (ELISA reader HLAB, model HReader1, HLab supply LTD).
Statistical analysis
The variables of follicular activity and serum P4 and E2 concentrations were analyzed using a univariate repeated measures ANOVA. Ovulation rate was analyzed through the Chi-square test. The probability of ovulation by treatment was analyzed through the Kaplan-Meier survival analysis. To know the effect of treatment on ovulation rate, follicular activity and serum P4 and E2 concentrations a logistic regression was used. All the statistical tests were from the SPSS (Statistical Package for Social Sciences V. 15).
Results and Discussion
A greater number of follicles and greater follicular diameter were found in cows from T3, compared to cows from T1, T2 and T4 (p<0.05) (Table 1).
Table 1 Number of ovarian follicles, follicle diameter and ovulation rate (mean ± SD) in Simbrah cows receiving or not feed supplementation, managed under continuous or restricted suckling.
| Variable | Treatment | |||
|---|---|---|---|---|
| T1 (CCC) n=30 |
T2 (RSC) n=30 |
T3 (RCC) n=30 |
T4 (CSC) n=30 |
|
| No. of follicles | 9.0 ± 1.0a | 11.0 ± 2.0b | 13.0 ± 2.0c | 7.0 ± 1.0d |
| Follicle diameter (mm) | 10.7 ± 0.1a | 12.6 ± 1.5b | 14.6 ± 2.3c | 8.0 ± 0.1d |
| Ovulation rate (%) | 100a | 100a | 100a | 100a |
a,b,c,d Different superscripts between columns by row indicate statistical difference (p<0.05).
T1= Continuous suckling with feed supplementation. T2= Restricted suckling without feed supplementation. T3= Restricted suckling with feed supplementation. T4= Continuous suckling without feed supplementation.
The results from this study indicate that restricted suckling, under the conditions of this experiment, did improve the reproductive efficiency of cattle. Cows from T2 (restricted suckling) had greater number of follicles, follicle diameter and serum P4 and E2 concentrations, in comparison with cows from T1 and T4 (continuous suckling). In addition, the effect of restricted suckling during early postpartum was potentiated when combined with feed supplementation; this was evident in the greater number of follicles, follicle diameter and serum P4 and E2 concentrations in cows from T3, in comparison with cows from T1, T2 and T4. Even though ovulation rate was 100 % in all the treatments (Table 1) because the study ended when all cows had ovulated, the Kaplan-Meier survival analysis indicated different time of ovulation by treatment, with higher probability of ovulation for cows from T3 than from the other treatments (p<0.05) (Figure 1).
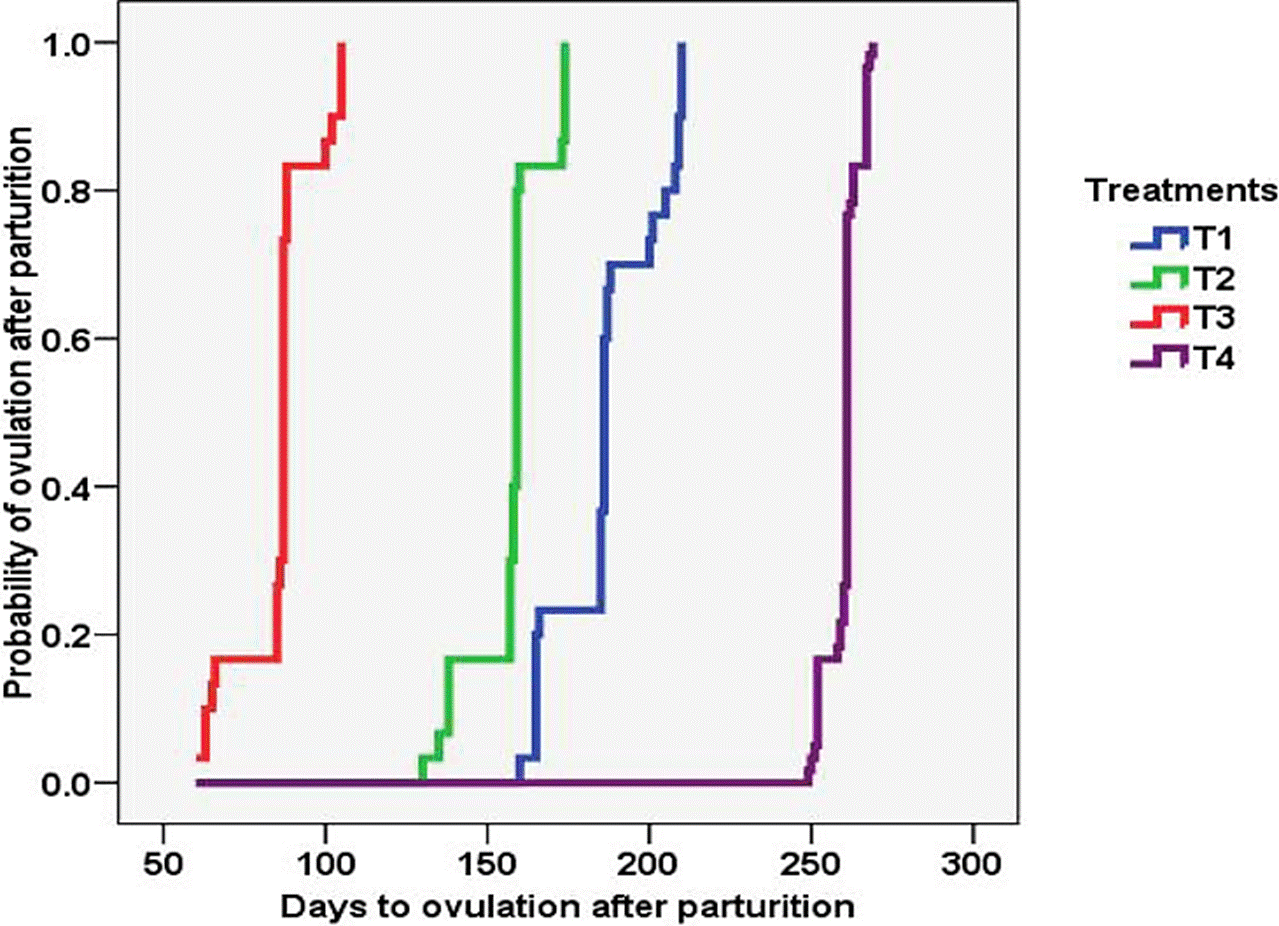
T1 = Continuous suckling with feed supplementation. T2 = Restricted suckling without feed supplementation. T3 = Restricted suckling with feed supplementation. T4 = Continuous suckling without feed supplementation.
Figure 1 Kaplan-Meier survival analysis indicating the probability of ovulation after parturition by treatment.
Blood serum concentrations of P4 (ng/mL) and E2 (pg/mL) were higher in cows from T3 than in cows from the other groups (Table 2).
Table 2 Serum progesterone and estradiol concentrations (mean ± SD) in Simbrah cows receiving or not feed supplementation, managed under continuous or restricted suckling.
| Variable | Treatment | |||
| T1 (CCC) n=30 |
T2 (RSC) n=30 |
T3 (RCC) n=30 |
T4 (CSC) n=30 |
|
| Progesterone (ng/ml) | 6.4 ± 2.1a | 7.6 ± 1.6b | 8.4 ± 1.1c | 5.8 ± 1.7d |
| Estradiol (pg/ml) | 50.4 ± 10.2a | 63.6 ± 12.7b | 70.7 ± 16.3c | 37.5 ± 7.5d |
a,b,c,d Different superscripts between columns by row indicate statistical difference (p<0.05).
T1= Continuous suckling with feed supplementation. T2= Restricted suckling without feed supplementation. T3= Restricted suckling with feed supplementation. T4= Continuous suckling without feed supplementation
If treatments are analyzed separately, restricted suckling combined with feed supplementation of the cows (T3) could be considered as the most convenient from a productive point of view. However, if it were not possible to implement restricted suckling in combination with feed supplementation, restricted suckling alone (T2) improves production and reproduction of cows, contrary to offering feed supplementation alone without separating the calf from the cow (T1), or neither separating the calf from the cow nor offering the cow feed supplementation (T4). To this respect, previous studies in cows (Arthington & Kalmbacher, 2003; Arthington & Minton, 2004; Schultz et al., 2005; Galindo-González et al., 2007; Quintans et al., 2009; Vittone et al., 2011; Martins et al., 2012; Waterman et al., 2012; Crowe et al., 2014; Lopes-Silva et al., 2015; Mondragón et al., 2016; Diskin & Kenny, 2016) have demonstrated that early weaning (EW), compared with continuous suckling (CS), improves weight gain (EW 491 ± 5.0 vs. CS 452 ± 5.0 kg), pregnancy rate (EW 95 vs. CS 65 %), ovulation rate (EW 100 vs. CS 75 %), body condition score (1-9 scale) (EW 6.34 ± 0.07 vs. CS 4.75 ± 0.07), number of follicles (EW 18 ± 2 vs. CS 12 ± 2), follicle diameter (EW 12.0 ± 0.14 vs. CS 8.0 ± 0.10 mm), serum concentrations of P4 (EW 5.0 ± 2.0 vs. CS 3.0 ± 1.0 ng/mL) and E2 (EW 110.0 ± 25.0 vs. CS 30.0 ± 15.0 pg/ mL) and shortens the postpartum anestrus period (EW 90 ± 10.0 vs. CS 134 ± 16.0 days).
In beef cattle raised under tropical conditions, suckling is one of the main factors affecting the postpartum anestrus period (Montiel & Ahuja, 2005; Quintans et al., 2010; Waterman et al., 2012). Early weaning or controlled suckling have been recommended as management strategies aimed to improve reproductive efficiency (Galina et al., 2001; Montiel & Ahuja, 2005). This was supported by the results from the present study, as restricted suckling was reflected as productive and reproductive improvements. However, the precise mechanism by which suckling alters the reproductive function is not completely understood yet (Montiel & Ahuja, 2005; Quintans et al., 2009; Mondragón et al., 2016). Following a normal calving (with no complications), an average of 30 days are needed for complete uterine involution and resumption of activity of the hypothalamic-hypophyseal-ovarian axis for the synthesis and release of gonadotropic hormones (Diskin & Kenny, 2016).
At calving, concentrations of P4 and E2 decrease to basal levels (≤ 0.5 ng/mL and ≤ 2 pg/mL, respectively), inhibiting their negative feedback (from luteal and placental origin) on the synthesis of follicle stimulating hormone (FSH) and luteinizing hormone (LH). In the following days, FSH is synthesized and released into the blood stream, resulting in a transitory increase (around 30 ng/ mL 3-5 days after calving); this occurs subsequently at 7-10 day intervals (Crowe et al., 1998, 2014). The first FSH increase stimulates the first surge of follicular growth after parturition, which in general results in the formation of a DF at 7-10 days after calving (Murphy et al., 1990; Savio et al., 1990; Crowe et al., 1993). On the other hand, the recuperation of the FSH reserves in the anterior hypophysis is slow, requiring 2-3 weeks to complete. During this time, circulating concentrations and the frequency of the LH pulse are low, and the first DF that forms does not ovulate. This occurs in all the cows, regardless they are being milked or suckled (Silveira et al., 1993; Griffith & Williams, 1996). Most of beef cows (85 %) are capable to ovulate at 25-35 days after parturition (Crowe et al., 1993; Duffy et al., 2000; Mackey et al., 2000; Crowe et al., 2014). However, this does not occur, because suckling prevents hypothalamic secretion of gonadotropin releasing hormone (GnRH), which is necessary for the synthesis of LH; this negative effect reduces the follicle development and its competence to ovulate a viable ovum (Quintans et al., 2009; Crowe et al., 2014; Mondragón et al., 2016).
In theory, this process occurs through the signal generated when the calf suckles and activates the opioidproducing neurons that have a neural association with the GnRH-producing neurons; this has a blocking effect, as it inhibits GnRH release and in consequence LH secretion (Pérez-Hernández et al., 2001; Crowe, 2008; Crowe et al., 2014). The effect of suckling becomes less potent as the postpartum period advances; namely, the mechanism that prolongs postpartum inactivity can be attributed to the cow-calf bond, as it has been observed that a cow that suckles its own calf maintains the pattern of suppression of LH release; on the contrary, if the cow suckles an unrelated calf, the LH pulses are reestablished normally like when ovulation is soon to occur (Williams et al., 1996; Stevenson et al., 1997). This can be explained through the theory that suckling increases the sensibility of the hypothalamus to the negative feedback of the estradiol produced by the ovary, resulting in low LH secretion; as the postpartum period advances, the GnRH pulse generator becomes less sensitive to the suckling stimulus, avoiding the effects of the estradiol negative feedback (García-Winder et al., 1984; Pérez-Hernández et al., 2001; Crowe et al., 2014). This results in increased LH pulsatile secretion, occurrence of a preovulatory LH peak and ovulation (Pérez-Hernández et al., 2001; Crowe, 2008). This can be achieved from day 30 after parturition with the aid of restricted suckling and/or temporary isolation (48 h) of the calf from its dam, resulting in an immediate (2-5 days) increase in LH pulsatile frequency, which stimulates ovulation of the first DF in up to 85 % of cows (Stagg et al., 1998; Sinclair et al., 2002).
Therefore, even though the route through which suckling affects the postpartum anestrus period has not been completely elucidated yet, the evidence indicates that during the postpartum period cows experience three physiological stages before resuming their reproductive functions: 1) uterine involution, which takes ≥ 30 days; 2) restoration of the hypothalamichyphophyseal-ovarian axis regarding the synthesis and release mainly of GnRH and LH, which occurs at 25-35 days after parturition; and 3) inhibition or decrease of the hypothalamic sensitivity and the maternal bond in order to restore the gonadotropin release, which can be induced through controlled suckling and/or temporary separation of the calf starting at 30 days after parturition.
In this study it was also observed that, independently of the effect of suckling on the resumption of the postpartum ovarian activity, the nutritional factor is also present; this was seen in cows from T3 compared to T1, T2 and T4. The resumption of the postpartum reproductive activity is also influenced by the nutritional status of the cows (Wettemann et al., 2003; Waterman et al., 2012; Diskin & Kenny, 2016). Inadequate nutrient intake after parturition will cause a severe decrease in the cow’s body fat reserves, affecting its reproductive performance by increasing the postpartum anestrus period and decreasing pregnancy rate (Diskin & Kenny, 2014, 2016).
From a biological perspective, in the early postpartum mammals favor lactation over fertility, as nutrient use is prioritized (Lucy, 2003). Because nutrients can be scarce in the first stage of lactation, the lactating cow utilizes its limited resources preferentially to assure the survival of the newly born calf, prioritizing the maintenance of the calf over the production of viable oocytes, because if one of these were fertilized and the resultant embryo implanted, the cow would have to deal with the physiological cost of establishing a new pregnancy (Wettemann et al., 2003; Granja et al., 2012). In other words, reproductive functions such as estrus cyclicity and establishment of a new pregnancy have low priority within the scale of nutrient utilization in the early lactating cow, and such functions will be activated only when the demand of nutrients for lactation, maintenance, growth and body reserves has been met (Wettemann et al., 2003; Granja et al., 2012; Watanabe et al., 2013).
Thus, duration of postpartum anestrus is initially due to the effect of suckling, at least during the first month after parturition. Later, the nutritional status of the cow will be responsible for the reproductive activity; therefore, a satisfactory reproductive performance will be obtained when cows have an optimal nutritional status (Wettemann et al., 2003; Granja et al., 2012; Diskin & Kenny, 2016). Nonetheless, the mechanism by which nutritional factors act on reproduction has not been determined yet (Wettemann et al., 2003; Diskin & Kenny, 2016). In cows induced to nutritional anestrus there is a decrease in plasma LH concentrations and an inhibition in the frequency of LH pulses as a result of hypothalamic reduction of GnRH secretion (Crowe et al., 2014; Diskin & Kenny, 2016). This has been attributed to an enhanced sensitivity of the hypothalamus to the negative feedback of estradiol from the ovary during feed restriction, which generates a decrease in the pulsatile GnRH and LH release (Richards et al., 1991; Crowe et al., 2014).
On the other hand, cows with food restriction release more LH in response to the intramuscular administration of GnRH, in comparison with cows in a moderate or high nutritional plane (Whisnant et al., 1985; Rasby et al., 1991). This suggests that the decrease or suppression in LH secretion in cows with food restriction does not result from lack of LH in the anterior hypophysis, but from the decrease in GnRH release. Although this process is not clear yet, it is assumed that there are metabolic factors that modulate the function of the GnRH pulse generator and the response of the hypophysis to it (Lents et al., 2008; Crowe et al., 2014; Diskin & Kenny, 2016). Different hypothesis try to explain how postpartum anestrus, follicle development and ovulation can be regulated by chemical substances of which blood concentrations vary according to the nutritional status of the animal (body condition or body fat reserves) and that act simultaneously as metabolic indicators to several sites of the hypothalamus-hypophysis-ovaries axis.
Some of these metabolic indicators are leptin (Ciccioli et al., 2003; Zieba et al., 2005), the insuline-like growth factor (IGF-I) (Richards et al., 1991; Diskin et al., 2003) and the non-esterified fatty acids (NEFA) (Salas et al., 2003; Wettemann et al., 2003). Some functions have been attributed to these indicators, such as those related to the control of the follicle development or the postpartum gonadotropin release (Funston, 2004; Lents et al., 2008), and they are likely important mediators between the effects of food intake and energy balance in cattle. Thus, serum levels of these hormones and growth factors that regulate such functions and their performance are affected by changes in body weight or in the nutritional status of the cows during the postpartum period (Diskin et al., 2003; Barb & Kraeling, 2004).
Therefore, it could be assumed that the duration of the postpartum anestrus is influenced or has interaction with several factors and/or physiological stages that must be overcome and/or manipulated in order to resume the reproductive functions: 1) uterine involution; 2) restoration of the hypothalamus-hypophysis-ovaries axis; 3) inhibition and/or decrease of the hypothalamic sensitivity and the maternal bond to restore the release of gonadotropins, and 4) maintenance of an adequate nutritional plane postpartum to send the body the metabolic signal that the conditions necessary to resume the reproductive activity are present.
Finally, the results from the present study allow to infer that, although the route through which controlled suckling, early weaning and feed supplementation aid to shorten the postpartum anestrus period is not clear, restricted suckling is a useful management practice to increase the reproductive performance of beef cows. Thus, it is necessary to consider the stages that postpartum cows must experience to control anestrus efficiently, and at the same time, keep conducting research focused on clarifying the routes by which temporary or permanent separation of the calf from its dam reactivate the reproductive process in the postpartum cow.











 texto en
texto en 


