Introduction
The World Organization for Animal Health (OIE), which has more than 150 member countries, considers that an animal presents welfare when it is healthy, comfortable, well fed, safe, is able to express its behavior, and does not suffer unpleasant states, such as pain, fear and anxiety (OIE, 2008). Unfortunately, the intensive production of animals for human benefit has reduced farms’ ability to provide animal welfare (AW). In the particular case of pigs, the high demand for the consumption of their meat generated an increase in their production and in the way they are housed during their production chain (USDA-FAS, 2019). In farms with semi-intensive and intensive management systems, pigs are constantly exposed to aversive events such as aggressive handling by farm workers (Tallet et al., 2018), early weaning (Siegford et al., 2008), formation of new groups or mix of animals of different ages (Colson et al., 2012), (Verdon et al., 2016), exposure to extreme temperatures (Parker et al., 2010), and housing with little or no social and sensorial stimulation (Merlot et al., 2012; Brajon et al., 2017). Studies indicate that this type of conditions generates stress in animals and, as a consequence, a poor welfare.
One of the serious problems of stress, understood as a response to a real or perceived threat altering the homeostasis of an organism (McEwen, 2000), is that it could have severe negative effects if it occurs in a prolonged or continuous way. Some of the physiological effects of stress include deficiencies in the immune system and the activation of the hypothalamo-hypophysis-adrenal (HPA) axis. While experiencing acute stress is an adaptive response with which an organism improves its capacity to face its environment and survive. Constant stress, prolonged or chronic, has negative effects on vital biological functions, such as reproduction, immune response and growth, mainly due to the quantity of glucocorticoids and catecholamines released in the system (Moberg, 2000).
In farms, stress negatively affects the exploitation of the animals since it reduces their health, causes a low productive yield (McLamb et al., 2013) (Dong and Pluske, 2007), alters their growth rates (Hyun et al., 1998), increases mortality rates (Edwards, 2002) and increases the incidence of undesirable behaviors (Manteuffel et al., 2004; Valros, 2018) (Holinger et al., 2018). A strategy that seems to mitigate the negative effects of stress and to increase AW, is the environmental enrichment (EE). Generally, it is defined as the environmental modification of captivity conditions, leading to an improvement in the biological performance of the animals (Ruth C. Newberry, 1995). One of the main objectives of the EE is to improve the way the animals face novelty and stress (Shepherdson, 1989). There are diverse types of EE, for instance social EE, including animal-animal and human-animal interactions, occupational EE, physical EE, involving the quality and quantity of available space, and sensorial EE, implying senses stimulation (Young, 2013). Studies in pigs indicate that EE increases the incidence of behaviors typical from the species, such as exploratory behavior (Yang et al., 2018) and playing behavior (Martin et al., 2015), while it decreases negative states such as aggressiveness (Marcet-Rius et al., 2019) and pre-natal stress (Brajon et al., 2017), in addition to fostering learning abilities (Jansen et al., 2009; Douglas et al., 2012).
Studies suggest that one of the main benefits of EE is that it helps animals to properly manage their emotions by making them more skillful to face stressful situations (Panksepp, 2004). In animals, emotions can be assessed by means of physiological and behavioral indicators, being these last ones the most practical. Considering that behavior evolved from basic skills allowing animals to survive, avoiding danger and searching for resources (Panksepp, 1982; Paul y Mendl, 2018). Emotions can be assessed by using behavioral paradigms, where individual behavior is registered and associated to emotional states, such as fear, anxiety, exploration and locomotion in a great number of animals, including pigs (Andersen et al., 2000; Wemelsfelder et al., 2000; Siegford et al., 2008; Donald et al., 2011; Rutherford et al., 2012; Tönepöhl et al., 2012).
Since pigs represent a relevant food source worldwide, it seems important to improve the knowledge on their basic emotional requirements and on the strategies driving the establishment of programs improving their welfare. Therefore, the present study evaluates the effect of EE on the capacity of pigs to respond to anxiety tests.
Material and Methods
Location and ethics
The study was performed in a semi-intensive pig meat farm located in the municipality of Emiliano Zapata, state of Veracruz, Mexico (19° 41′ N, 96° 56′ W), with an altitude of 165 meters above sea level, annual average temperature of 25.2 °C and annual precipitation of 2700 mm (García, 1988).
The use of animals and the procedures to which animals were submitted during the study were approved by the Commission of Bioethics and Animal Welfare of the Faculty of Veterinary Medicine and Zootechny of the University of Veracruz.
Experiment 1. Effect of EE on piglets performance in novel object test and elevated plus maze
Animals
20 F1 sows (Yorkshire/Landrace) with 3 to 4 parturitions were selected, and a score of 3.5-4 for body condition in a scale of 5 points (1=thin and 5=obese) (Gadd, 2011), whose offspring were used. Sows were randomly divided into a treatment group (n = 10) and a control group (n=10). From 7 to 15 weeks of pregnancy, sows were housed in individual cages of 2.5 m in length and 0.80 m in width, equipped with a water dispenser, a semiautomatic eating trough and grooved cement floor. Posteriorly, from 15 to 19 weeks of pregnancy, sows were housed in cages of 2.5 m in length and 1.2 m in width, each one equipped with a classic eating trough, water dispenser and raised grid floor of 1 m high. In the front part, the cages had a wooden pig breeding center to provide refuge and heat to the offspring by means of an electric heater. Food for the pregnant sows consisted in a daily portion of 1.5 kg of feed concentrate (soy, sorghum or corn, vitamins, minerals and some additives) provided in one dose, while sows in maternity were provided with 7 to 10 kg of food daily, divided into four portions. On the other hand, food for the offspring consisted in breastmilk ad libitum plus 100 mL of pre-initiator feed (Apligain pig, Apligén ®), given from the second week of life and until weaning.
Environmental enrichment for pregnant sows
The occupational enrichments used for the sows during pregnancy were the following:
Homemade food dispenser: 4-inch polyvinyl chloride (PVC) tubes of 50 cm in length, with 16 holes of approximatively 1 cm in diameter, distributed to its full extent, with lids at both ends. The tube was placed horizontally, fastened at the front of the cage, acting as a roller, which the sows could make spin around to obtain 100 g of pre-initiator feed for offspring, and which was placed before each EE.
Rope: Three segments of polypropylene-polyester plaited rope of 20 cm in length and 6 mm in diameter were used, fastened to the cage.
Plastic tube: It consisted in 3 segments of hose of 30 cm in length and 1 inch in diameter, united to a cotton rope of 40 cm in length allowing to fasten it to the cage.
Straw: 300g of straw from Pangola-grass (Digitaria decumbens) was provided, cut into 5 cm in length approximately and placed in the trough of each sow.
Straw bed: Straw of 20 cm in length was provided, distributed on all the floor of the cage, making a bed of 1-2 cm thick.
Fresh coconut shell: Freshly cut coconuts were used, halved and drilled, a rope of 40 cm in length was introduced through the hole to hang it in the cage.
Massages: A tactile stimulation was provided on the back and laterals of the sows using a waterproofing roller covered with synthetic grass (BYP®), multi-purpose work gloves (Skin-dex®), or floor scrub brushes (Thermotek®).
Exposure of sow to EE
The sows from the treatment group were exposed for 9 weeks to one of the different types of enrichment, both occupational and sensorial, while the sows from the control group were not submitted to any type of enrichment. While the occupational stimuli were provided for 30 minutes each, the sensorial ones were provided for 5 minutes with intervals of 1 minute of stimulation and 1 minute of rest, with a movement pattern from top to bottom (from head to tail). Every day, the sows were exposed to one of the different types of stimuli, on a schedule from 9 a.m. to 12 p.m. and performed by the same person.
EE for piglets
The enrichment provided to the piglets consisted in:
Massage: with a multi-purpose work glove (Skin-dex®) or with a floor scrub brush (Reynera®).
Ball: five unfilled plastic balls (payaso®) of approximately 5-10 cm in diameter were used as a toy for children and placed on the floor of the cages.
Plastic tube: segments of industrial hose of 15 cm in length and ½ inch in diameter were placed in the cage, fastened by means of a segment of rope of 40 cm in length as a pendant.
Rope: similar to the one used for the sows.
Wood: wooden segments of 20 cm in length x 5 cm in width, joined together forming a star shape, fastened with a rope of 40 cm in length.
Exposure of piglets to EE
After birth, 2 piglets of each sow from the treatment group were exposed for 21 days to the different types of enrichment mentioned in the previous section. The massages were provided during the first week of life of the piglets, for 30 seconds daily, with a movement pattern from top to bottom (from head to tail) with an approximate duration of 1 second per movement. On the other hand, the occupational stimuli were provided one per day, for 30 minutes, during the second and third week of life. The piglets were exposed to one of the different types of stimuli, on a schedule from 9 a.m. to 11 a.m. The piglets of the sows from the control group were not submitted to any additional stimulation except the one provided during the typical handling of the farm.
Exposure to behavioral tests
On post-natal day 22, the piglets were exposed to the novel object (NO) test and on day 23 to the elevated plus maze (EPM) test.
Novel object test
NO test is used to assess fear or anxiety responses to the lack of familiarity with the performed stimuli (Boissy, 1995). Animals suffer a conflict between their motivation to explore and the avoidance when they are faced to a novel object (Adaptado de Tönepöhl et al., 2012).
In the present study, each pig was individually exposed and placed inside a wooden quadrangular crate of 2 x 2 x 1 meters, with a door of 0.80 x 0.80 in one of its sides, floor covered with wood with a sketch of 9 squares of 66.6 x 66.6 cm. Previous to the entry of each pig, a donkey-shaped inflatable toy for children of 0.60 x 0.50 cm was placed in the crate (novel object). Each pig was free of moving on the arena for 10 minutes. The number of squares crossed, latency of first NO exploration, frequency of NO exploration, duration of NO exploration, latency of first escape attempt, frequency of escape attempt (when the animal tries to leave the test, jumping over the walls of the arena), latency of first vocalizations, frequency of vocalizations, latency of first urination, frequency of urination, latency of first defecation and frequency of defecation were registered.
Elevated plus maze test
A cross-shaped elevated plus maze (EPM) was used, based on the one described by Andersen et al., (2000). This paradigm has been widely used in rodents to evaluate the anxiolytic properties of determined stimuli and medications, and recently it has been used in other species such as pig for the same purpose. The EPM was made of four arms, two opposed open arms without walls (125 x 0.50 cm) and two closed arms with walls (125 x 0.50 x 0.50 cm3) united by a central platform (0.50 x 0.50 cm2). The device was raised at 1 m height over the concrete floor of the test corral. Each pig was placed individually in the middle of the device for a period of 5 minutes. The frequency, latency and duration on open and closed arms, frequency and latency of attempts of entry into open and closed arms were registered, as well as the frequency and latency of risk assessment (number of times the animal attempts to leave the test, placing itself in a posture of jump attempt). If the animal leaves the ring, its assessment terminated.
Experiment 2: Effect of EE in different developmental stages of piglets on their performance in elevated plus maze test
Experimental animals and handling
Sixty piglets were selected from the day of their birth and were divided into four groups: animals from group 1 were offspring from sows who were provided with EE from day 70 of pregnancy (pregnancy), and who were later provided with EE during the 21 days after the birth (lactation). On the other hand, group 2 were piglets who were provided with EE during their weaning (Post-natal days 22 to 70), those from group 3 were provided with EE during pregnancy-lactation-weaning and those from group 4 were animals who were never provided with EE.
During lactation, piglets were housed with their mothers in the maternity area. During weaning, piglets were separated from the mother and housed in groups of 15 in corrals of 3.5 m in length and 2 m in width, equipped with two water dispensers, a semi-automatic trough and raised grid floor and cement lower floor with a slope where they were fed with commercial feed concentrate according to the developmental stage (pre-initiator, step 1, step 2 and step 3), at the rate of 1.2 to 1.7 kg/head/day.
Exposure to EE
The EE during the lactation period was the same as described in the previous experiment in the section “EE for piglets.” On the other hand, the EE in weaning consisted in: All EE used in experiment 1.
Cones: four orange traffic cones of 30 cm high.
Drums: 3 empty rectangular 5L drums.
Each stimulus was provided into the corrals during 30 minutes. Piglets interacted with the EE once a day on a schedule from 9 a.m. to 11 a.m.
Elevated plus maze test
On post-natal day 71, each piglet was individually exposed to the EPM test. Since these animals were much bigger than those from the previous experiment, the dimensions of the maze were increased to 2.5 x 1 m for each open arm and to 2.5 x 1 x 1 m3 for each closed arm with walls. After placing piglets in the central area of the EPM and allowing them to explore it for the same period of time as in the previous experiment, the same behaviors as described in the previous experiment were registered.
Statistical analysis
In experiment 1, a Student t-test was performed on independent samples to compare the means of behaviors between groups with and without EE during their exposure to NO and EPM tests. In experiment 2, a one-way analysis of variance (ANOVA) was performed with STATISTICS version 10 software. To identify significant differences among groups, a post hoc Tukey’s test was performed. Alfa value for all comparisons was p < 0.05.
Results and discussion
Experiment 1
The statistical analysis indicated that, during the NO test, the piglets from the group without EE presented a higher number of escape attempts and vocalizations, as well as a lower latency of first vocalization (p < 0.05). Total behavioral parameters assessed during the NO test were shown in Table 1.
Table 1 The behavioral parameters of piglets assessed during the novel object test (NO) in Experiment 1.
| Behavioral parameters | Group with EE | Group without EE |
| Number of squares crossed | 54 ± 4.0 | 57 ± 4.7 |
| Latency of 1st NO exploration (s) | 66.8 ± 18.3 | 58.8 ± 14.0 |
| Frequency of NO exploration | 5.3 ± 0.5 | 6.2 ± 0.6 |
| Duration of NO exploration (s) | 17.8 ± 3.7 | 18.7 ± 3.2 |
| Latency of 1st escape attempt (s) | 44.7 ± 18.0 | 88 ± 21.6 |
| Frequency of escape attempt | 0.8 ± 0.3* | 2.9 ± 0.9 |
| Latency of 1st vocalization (s) | 91.2 ± 17.0* | 24.3 ± 5.7 |
| Frequency of vocalization | 143 ± 23.4* | 242 ± 35.0 |
| Latency of 1st urination (s) | 28.9 ± 15.8 | 14.2 ± 14.2 |
| Frequency of urination | 0.2 ± 0.1 | 0.0 ± 0.0 |
| Latency of 1s defecation (s) | 51.7 ± 19.4 | 37.2 ± 15.4 |
| Frequency of defecation | 0.4 ± 0.1 | 0.3 ± 0.1 |
Latencies and duration were expressed in seconds (s). Mean ± standard error of the mean (SEM).
* Indicated significant differences among groups, p < 0.05.
Regarding the EPM test, the Student t-test identified that piglets from the group with EE showed a lower latency of first attempt to enter into open arms and a higher frequency of risk assessment, compared with piglets from the group without EE (p < 0.05). Total behavioral parameters assessed during the EPM test were shown in Table 2.
Table 2 The behavioral parameters of piglets assessed during the elevated plus maze test in Experiment 1.
| Behavioral parameters | Group with EE | Group without EE |
| LECA (s) | 11.3 ± 4.4 | 9.5 ± 2.9 |
| FECA | 1.2 ± 0.3 | 2.1 ± 0.5 |
| DCA (s) | 13.6 ± 4.5 | 25.5 ± 6.9 |
| LEOA (s) | 23.0 ± 3.4 | 29.6 ± 6.4 |
| FEOA | 1.9 ± 0.5 | 2.0 ± 0.3 |
| DOA (s) | 33.4 ± 4.6 | 27.4 ± 4.6 |
| LAECA (s) | 4.7 ± 4.7 | 4.9 ± 2.9 |
| LAEOA (s) | 6.4 ± 2.6* | 19.5 ± 5.9 |
| FAEOA | 0.3 ± 0.1 | 1.0 ± 0.3 |
| LRA (s) | 35.2 ± 3.3 | 33.8 ± 4.5 |
| FRA | 3.5 ± 0.5* | 5.9 ± 0.8 |
Latencies and durations were expressed in seconds (s). Mean ± standard error of the mean (SEM).
* Indicated significant differences among groups, p < 0.05.
LECA: Latency of 1st entry into closed arms; FECA: Frequency of entries into closed arms; DCA: Duration on closed arms; LEOA: Latency of 1st entry into open arms; FEOA: Frequency of entries to open arms; DOA Duration on open arms; LAECA: Latency of 1st attempt to enter into closed arms; FAECA: Frequency of attempts to enter into closed arms; LAEOA: Latency of 1st attempt to enter into open arms; FAEOA: Frequency of attempts to enter into open arms; LRA: Latency of risk assessment; FRA: Frequency of risk assessment.
Experiment 2
The analysis of variance detected significant differences for the duration in open arms among groups F(3, 56) = 3.74, p < 0.05). The post hoc test indicated that piglets enriched during pregnancy-lactation remained in open arms during longer periods compared with those that were provided with EE during pregnancy-lactation-weaning and with those that were not provided any EE (Figure 1). Regarding the latency of risk assessment F(3, 56) = 2.85, p < 0.05), the post hoc test indicated that piglets enriched during pregnancy-lactation had a higher latency of risk assessment compared with the rest of the groups (Figure 2). Finally, the statistical test showed significant differences for the frequency of risk assessment among groups (F (3, 56) = 3.95, p < 0.05). The post hoc test indicated that piglets enriched during pregnancy-lactation assessed a higher number of abandonments of the test, contrary to piglets enriched during pregnancy-lactation-weaning and those without EE (Figure 3). Total behavioral parameters assessed during the EPM test were shown in Table 3.
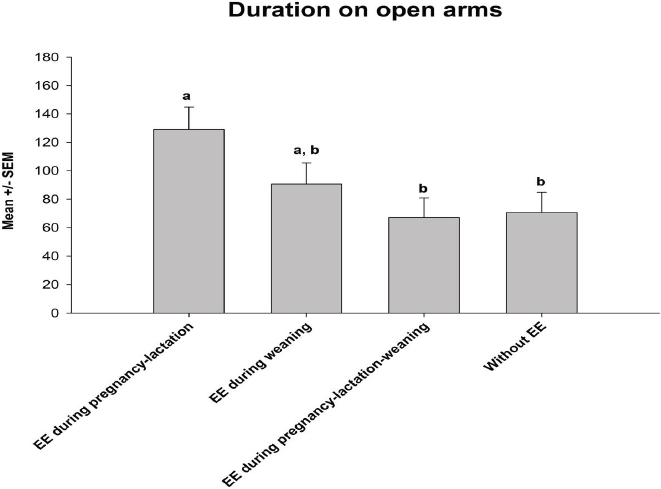
Figure 1 Duration on open arm. Data were shown as mean ± standard error of the mean (SEM). Different superscript letters indicated significant differences, p < 0.05.
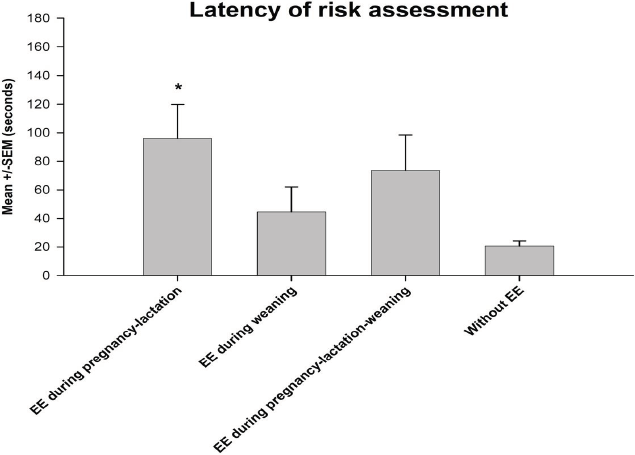
Data were shown as mean ± standard error of the mean (SEM). * Indicated p < 0.05.
Figure 2 Latency of risk assessment.
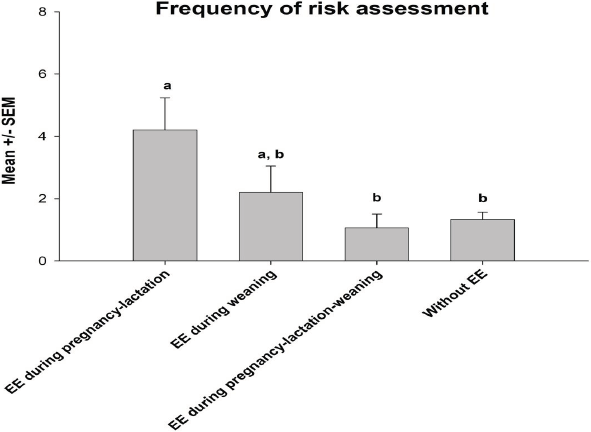
Figure 3 Frequency of risk assessment. Data were shown as mean ± standard error of the mean (SEM). Different superscript letters indicated significant differences, p < 0.05.
Table 3 The behavioral parameters of piglets assessed during the elevated plus maze test in Experiment 2.
| Groups | ||||
|---|---|---|---|---|
| EE during pregnancy-lactation |
EE during weaning |
EE during pregnancy-lactation-weaning |
Without EE |
|
| LECA (s) | 60.1 ± 14.7 | 47.9 ± 14.7 | 45.4 ± 14.7 | 70.5 ± 14.7 |
| FECA | 3.0 ± 0.3 | 3.6 ± 0.3 | 3.7 ± 0.3 | 3.0 ± 0.3 |
| DCA (s) | 68.1 ± 15.4 | 107.8 ± 15.4 | 118.3 ± 15.4 | 110 ± 15.4 |
| LEOA (s) | 29 ± 13.5 | 46.5 ± 13.5 | 49.4 ± 13.5 | 30 ± 13.5 |
| FEOA | 4.6 ± 0.5 | 3.4 ± 0.5 | 2.5 ± 0.5 | 2.8 ± 0.5 |
| DOA (s) | 129 ± 14.6* | 90.7 ± 14.6 | 67 ± 14.6 | 70.6 ± 14.6 |
| LAECA (s) | 87.3 ± 25.3 | 96.1 ± 25.3 | 116.8 ± 25.3 | 89.6 ± 25.3 |
| FAECA | 1.1 ± 0.3 | 1.4 ± 0.3 | 1.0 ± 0.3 | 1.2 ± 0.3 |
| LAEOA (s) | 71.2 ± 20.8 | 83.9 ± 20.8 | 96 ± 20.8 | 89.2 ±20.8 |
| FAEOA | 1.0 ± 0.2 | 1.4 ± 0.2 | 1.3 ± 0.2 | 1.8 ± 0.2 |
| LRA (s) | 95.8 ± 19.4* | 44.4 ± 19.4 | 73.5 ± 19.4 | 20.6 ± 19.4 |
| FRA | 4.2 ± 0.7* | 2.2 ± 0.7 | 1.0 ± 0.7 | 1.2 ±0.7 |
| LU (s) | 32.4 ± 21.5 | 64 ± 21.5 | 38.8 ± 21.5 | 23 ±21.5 |
| FU | 0.1 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| LD (s) | 72.8 ± 19.5 | 55.4 ±19.5 | 35.8 ± 19.5 | 76.8 ± 19.5 |
| FD | 1.1 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 |
Latencies and durations were expressed in seconds (s). Mean ± standard error of the mean (SEM). * Indicated significant differences among groups, p < 0.05.
LECA: Latency of 1st entry into closed arms; FECA: Frequency of entries into closed arms; DCA: Duration on closed arms; LEOA: Latency of 1st entry into open arms; FEOA: Frequency of entries to open arms; DOA Duration on open arms; LAECA: Latency of 1st attempt to enter into closed arms; FAECA: Frequency of attempts to enter into closed arms; LAEOA: Latency of 1st attempt to enter into open arms; FAEOA: Frequency of attempts to enter into open arms; LRA: Latency of risk assessment; FRA: Frequency of risk assessment; LU: Latency of 1st urination; FU: Frequency of urination; LD: Latency of 1st defecation; FD: Frequency of defecation.
In this study, the effect of EE on the response of infantile and juvenile piglets was evaluated during the exposure to behavioral paradigms assessing stress-related emotions, anxiety and fear. Results showed that piglets whose mothers were provided with enrichment during their pregnancy and were later stimulated during the lactation period presented a lower number of anxiety-related behaviors compared with piglets that were never provided with EE. In accordance with these first findings, results from the second experiment showed that being provided with EE during this same period improved the response of piglets while facing stress at the juvenile stage. This indicated that pre- and neo-natal periods represented one of the most crucial periods in the emotional development of piglets and that theoretically positively impacted their capacity to face aversive situations.
The EPM test has been widely used to evaluate anxiolytic effects of diverse substances (Pellow et al., 1985; Wilson et al., 2004; Walf y Frye, 2007). Its principle stems from the assumption that animals naturally find aversive entering or staying in open and elevated spaces, thus the anxiety generated by this paradigm results in behavioral indicators such as the frequency of entries in open arms and the duration of presence on them, since under these conditions, the animals feel defenseless and exposed to potential threats. In this study, piglets without EE were observed to present a lower number of attempts to enter into open arms and to assess more opportunities to leave the test compared with piglets that were provided with EE. In addition, in the second experiment, piglets that were provided with EE during the same period and during their weaning improved their performance during the EPM test when they were 71 days old. Studies have demonstrated that early handling of animals including tactile stimulation has positive effects on their physical and psychological development (Fernández-Teruel et al., 2002). On the other hand, it has been observed that in rodents and other species, the pre- and post-natal periods are crucial steps in their development (Otten et al., 2015). For example, it is known that adverse experiences, such as stress during pregnancy, caused complications in offspring at birth, low weight at birth, reduced litter size and lower survival rates. In addition, these animals tend to experience higher anxiety, abnormal social behaviors, increase in the HPA axis activity and cognitive deficits in adulthood (Lee et al., 2007; Paris y Frye, 2011; Buss et al., 2012). On the other hand, early post-natal stimulation (neo-natal handling) and the EE produce deep and long-lasting behavioral and neurobiological effects affecting the capacity of individuals to explore, regulate their emotions, learn and in motor and cognitive abilities in adulthood (Renner y Rosenzweig, 1987; Fernandez-Teruel et al., 1997). The EE stimulates neurogenesis, survival, differentiation and growth of neurons in the hippocampus, a fundamental region for spatial and episodic memory of animals (Birch et al., 2013), and fosters the maturation of the visual system through sensory experience (Baroncelli et al., 2009). Studies suggested that tactile stimulation in early developmental stages generates small dose of stress in animals, accelerating the maturation of the central nervous system (Levine, 2005), modifies the number of glucocorticoids receptors in the hippocampus and increases the neuroplasticity (Champagne et al., 2009). Since neurogenesis and neuroplasticity are directed to individual biological adaptation (Panksepp, 2004), it is thought that the effect of EE on the central nervous system of the animals aims to make individuals more able to cope with stress, and less prone to experience negative affective states as fear, anger, anxiety and depression for long periods of time (Panksepp, 2010).
In the case of pigs, studies indicated that individuals that were provided with EE during the lactation period increased the frequency of play and exploration behaviors (Held y Špinka, 2011), while it decreased anxiety responses when facing novel events (Wemelsfelder et al., 2000). In this study, the behavior presented by pigs during their exposure to the EPM and NO tests were considered to be the result of an emotional conflict between experiencing fear of open environments (active avoidance) and the motivation for exploring a novel environment (Murphy et al., 2014). Reluctance to enter into open arms has an ecological relevance that is associated with an innate aversive behavior of agoraphobia and fear of falling (Carobrez y Bertoglio, 2005). The results of the first experiment during the EPM tests suggested that pigs with EE did not show an unconditioned avoidance to perform attempts to enter into open arms since their latency were lower than those of individuals without EE. Unfortunately, there was no group that was provided EE only during pregnancy or only during lactation, therefore it was not possible to determine whether there were differences among them, or whether one of these periods is more important than the other.
Regarding the NO test, piglets reared in common environment (without enrichment) were observed to present a higher number of active avoidance behaviors, suggesting that the novel object used in this study generated similar responses to those that a prey presents when facing a predator (Gray, 1987; Blanchard, 1997; Forkman et al., 2007). From an evolutionary point of view, this defensive reaction allows the animals to keep safe from threats, and is probably activated when the animal is faced to situations representing a threat, such as interactions with humans, changes in its physical and social environment or aversive situations causing fear-related responses (Forkman et al., 2007; Blanchard y Blanchard, 2008). However, an exaggerated or decontextualized response of pigs towards an inert object suggested that individuals without stimulation did not regulate their emotions during changes in their environment and did not adequately handle novelty, generating a higher stress, fear and anxiety. In farms, pigs are constantly exposed to aversive situations, such as changes of corral, application of injections, ear tagging, among others. Therefore, the present data suggested that the EE can help pigs to reduce their stress-, fear- and anxiety-related response during their time in the production chain.
Moreover, pigs without EE were found to perform a higher number of vocalizations while exposed to the NO test. In highly vocal animals as pigs, vocalizations have been found to be associated to a great number of emotional responses (Leliveld et al., 2016), for example, they play a very important role in individuals recognition (Weary y Fraser, 1995; Melišová et al., 2011), during feeding (Jensen y Algers, 1984; Ruth C Newberry y Wood-Gush, 1985), hierarchical organization (Drake et al., 2008), pain (Weary et al., 1998; Leslie et al., 2010), transport (Brandt y Aaslyng, 2015) and social isolation (Marchant et al., 2001). Nevertheless, increases in its frequency are associated with high levels of excitation (elevated sympathetic-adrenomedullar stimulus), like when an individual is isolated from its group and is exposed to a novel environment (Herskin y Jensen, 2000). Findings of the present study suggested that the reduction in the quantity of anxiety-and fear-related signs in animals enriched during pregnancy and lactation periods implicates a better capacity to regulate their emotions during aversive situations.
Among agricultural activities, pig farming is one of the most intensive production systems, in which the exploitation of animals is successfully maximized at low cost. Although this study did not consider productive parameters, such as weight gain, litter weight at weaning and daily food intake, stress is known to decrease food intake and feed conversion rate (Figueroa et al., 2013), hence, it can be speculated that if animals are able to cope with stress and are in a welfare condition, they will have a higher feed intake and consequently, higher productive indicators. Further studies on AW, ethology and stress in animals intended for human consumption should consider the effect of EE on production, which would very likely encourage producers and entrepreneurs to implement such programs in pig farms (Van de Weerd & Day, 2009).
Overall, results of the present study indicated that the EE during pregnancy and the first weeks of life can improve the capacity of pigs to face novel and stressing situations, such as those they face throughout their lives in most of farms around the world. One of the most relevant findings of this study was the identification of the pre- and neo-natal periods as ones of the most beneficial for emotional development in pigs. This has a great relevance and application for farms, since it represents a practical, inexpensive and science-based approach to improve pig welfare during the production chain. However, a higher number of studies is required to determine whether the effect of EE during these stages persists lifelong and whether it has any effect on its productive parameters, as well as on the activation of the HPA axis and glucocorticosteroids levels, mainly cortisol, and on the activity of the immune system, both at cellular and humoral scales.
Conclusion
Results of the present study showed that piglets reared in enriched environments during pre- and neo-natal periods presented a better performance in the NO and EPM tests compared with piglets reared in typical production environments (without EE). Similarly, this study revealed that the EE during this same period had a greater benefit compared with enrichments after the weaning. In conclusion, this study indicated that the EE is a good strategy to improve AW in farmed pigs.











 texto en
texto en 


