Introduction
Most of the worldwide diseases in crops are mainly caused by fungi, bacteria, viruses, and nematodes. These are responsible for large economic losses by the decrease of the quality of the product or the total loss of the crop when the severity is high. However, fungi are the major cause of crop damage in agricultural activities (Rios et al., 2016).
The management of diseases in crops depends on the application of chemical fungicides (APEAM, 2019). Some of them are known for their negative impacts on the environment and even on human health, as well as in the development of fungicide-resistant strains of fungal plant pathogen due to the strong selection pressure and to the loss of biodiversity in agroecosystems. Given the social pressure to obtain safe food, the search for alternatives for crop disease management has been intensified (Fisher et al., 2012; Gerbore et al., 2014). Cultural and genetic alternatives stand out among the available options, and recently, the use of biological control agents with emphasis on native antagonistic microorganisms such as bacterial strains (Zeilinger & Omann, 2007; Andrade et al., 2015) as sustainable alternatives in disease management (Ait-Lahsen et al., 2001). Among the antagonistic microorganisms, bacteria such as Pseudomonas, Burkholderia, Acinetobacter, Arthrobacter, Azospirillum, Serratia and Bacillus (Suárez & Rangel, 2013; Zavaleta et al., 2015), are a promising alternative due to their antagonistic ability against a wide variety of phytopathogens because of the production of various antimicrobial compounds and for the multiple positive effects on plants as promoters of plant growth (Kloepper et al., 2004; Carreras, 2011; Pliego et al., 2011).
The species of the genus Bacillus are the most widely distributed and studied due to their antagonistic capacity against a great variety of phytopathogens (Pozo et al., 2007; Leelasuphakul et al., 2008). The bacteria have been isolated from different habitats, generally associated with the rhizosphere of plant tissues, where B. megaterium, B. licheniformis, B. circulans and B. subtilis stand out. B. subtilis is the most commonly used as a phytopathogenic antagonist (Cazorla et al., 2007; Tendulkar et al., 2007; Ali et al., 2009; Mehta et al., 2010; Rios et al., 2014). Although the urgent need for new alternatives in Mexico exists, few commercial products for the biological control of phytopathogens can be found, and that fact that whether the antagonistic microorganisms from these products are native or exotic is mostly unknown (Zavaleta et al., 2015). Therefore, the objective of the study was to identify and evaluate the in vitro antifungal activity of bacterial isolates against fungi of economic importance for Mexico.
Material and methods
Location of the experimental site.
The antagonistic bacteria were isolated from soil samples (500-600 g), associated with the rhizosphere of Hass avocado trees (10-15 cm depth). Sampling was carried out from January to December 2014 (Rios et al., 2014), in 20 commercial orchards in the municipalities of Tepic and Xalisco, Nayarit, Mexico (Table 1). The samples were transferred in polyethylene bags (Ecobags® 20 × 30 cm) to the Agricultural Parasitology Laboratory of the Multidisciplinary Center for Scientific Research (CEMIC) of the Universidad Autónoma de Nayarit (UAN) and stored at 4 °C, until further use.
Table 1 The geographic location of avocado orchards of the municipalities of Nayarit, Mexico.
| Municipalities | |||||
|---|---|---|---|---|---|
| Xalisco | Tepic | ||||
| Locality | Geographic location |
Altitude (mamsl) |
Locality | Geographic location |
Altitude (mamsl) |
| Emiliano Zapata | 21°22'50" N 104°56'01" O |
1,191 | Camichin III | 21°29'57" N 104°46'39" O |
1,022 |
| Curva | 21°22'11" N 104°53'38" O |
1,033 | Camichin II | 21°29'31" N 104°47'22" O |
1,006 |
| Xalisco | 21°26'00" N 104°54'11" O |
1,002 | Libertad | 21°31'44" N 105°01'29" O |
1,008 |
| Tacote | 21°24'46" N 104°55'35" O |
1,129 | Yerba | 21°31'27" N 105°02'45" O |
850 |
| Cuarenteño | 21°27'49" N 105°01'38" O |
1,118 | Guayabitos | 21°31'11" N 104°59'51" O |
1,120 |
| Carbonera | 21°27'40" N 105°00'28" O |
1,423 | Venustiano Carranza | 21°31'00" N 104°58'58" O |
1,099 |
| Xalisco U.A.A. | 21°25'32" N 104°53'31" O |
977 | Ahuacate | 21°31" 03" N 104°56'26" O |
978 |
| Tezcalate | 21°31'44" N 105°01'29" O |
1,030 | Fortuna | 21°32'47" N 104°56'33" O |
862 |
| Comunidad Indígena |
21°23'35" N 104°53'46" O |
1,027 | Tintilagua | 21°29'02" N 104°46'38" O |
1,148 |
| Pantanal | 21°25'54" N 104°52'16" O |
952 | Noria | 21°30' 33" N 104°58'20" O |
1,254 |
* mamsl: meters above sea level.
Isolation and identification of bacteria.
For the isolation and identification of bacteria, the soil samples from each orchard were homogenized and a 10 g sub-sample was taken, placed in 90 mL of sterile distilled water, kept under constant stirring for 30 min. Subsequently, a 1 mL aliquot was taken and placed in a test tube with 9 mL of sterile distilled water. Serial dilutions until 10-3, 10-4 and 10-5 were performed, and 80 µL were taken and deposited in duplicate in 90 mm diameter Petri dishes containing Papa Dextrose Agar culture medium (PDA) and then homogenized using the diffusion technique (Guigón & González, 2004). Petri dishes were incubated at 28 °C until bacterial growth was observed. Bacterial colonies that showed inhibition against fungi or other bacteria that grew in Petri dishes were isolated and grew in PDA at 48 h at 28 °C. Bacterial identification was carried out by analyzing the sequence of the 16S rDNA region. To perform this, genomic DNA (gDNA) of the bacteria was extracted with the ZR Fungal/bacterial DNA Miniprep ™ (Zymo Research, Irving CA, USA) commercial kit based on the manufacturer’s instructions. The quality and quantity of DNA were determined by spectrophotometry at O.D. 260 nm The integrity of the DNA was evaluated by electrophoresis in 1 % agarose gels. The 16S ribosomal region of each of the bacterial isolates was amplified by PCR, using primers FD1 (5´-TCGTCGACAACAGAGTTTGATCCTGGCTCAG-3´) and RD1(5´-CCCGGGATCCAAGCTTAAGGAGGTGAT CCAGCC-3´).
The PCR amplification conditions were carried out at 94 °C for 2 min (1 cycle), 94 °C for 30 s, 55 °C for 30 s, and 72 ° C for 1.5 min (35 cycles), with a final extension at 72 °C for 5 min. The amplified PCR products were purified with the Gen Elute PCR Clean-up kit (Sigma, CA, USA) according to the manufacturer’s instructions. The sequencing was done by the Institute of Biotechnology UNAM (IBT-UNAM, Morelos, Mexico). The sequences of the bacterial isolates were compared with the Ribosomal Database Project (RDP) and NCBI GenBank databases using BLAST to verify the percentage of identity corresponding to the species.
In vitro assays of antifungal activity.
The antagonistic capacity of the 10 isolated bacterial strains of avocado rhizosphere against Phytophthora cinnamomi, Fusarium oxysporum and Colletotrichum sp., fungi obtained from the microorganisms collection of the CEMIC, were evaluated by means of in vitro confrontations by a diffusible medium with the four-quadrant test. The isolates of B. methylotrophicus (Bm-10) and B. amyloliquefaciens (Ba-01) included in these assays were provided by the Centro de Investigación en Alimentación y Desarrollo A.C. These strains have been previously reported with potential for the control of phytopathogenic fungi. (Rios et al., 2014).
The confrontations of bacteria against fungi were carried out according to the methodology described by Rios et al. (2014) with modifications. Five mm diameter fungus disks (unquantified conidia) of eight days of growth were placed in the center of the Petri dish containing PDA. The antagonistic bacterium was inoculated the same day in the four cardinal points of the Petri dish, with disks of PDA of the same diameter of fungi with bacterial growth of 72 h. The controls consisted of a 5 mm diameter fungus disk in PDA in the absence of bacterial isolates.
The Petri dishes were incubated at 28 °C in a bioclimatic chamber (Novatech Ei45) and systematic measurements were made every 24 h for 10 d of the radial growth of the fungi colonies and antagonistic bacteria in confrontation and the controls. The antifungal capacity of bacterial isolates was determined based on the percentage of radial growth inhibition (PIRG) according to the PIRG formula = (R1 - R2) / R1 × 100, where R1 was the radius of the control fungus and R2 was the radius of the fungus in the confrontation with the antagonists (Ezziyyani et al., 2004).
A completely randomized design was used for the statistical analysis. The experiment consisted of 12 treatments (isolated from antagonistic bacteria), confronted with three putatively phytopathogenic fungi, besides of control for each fungus, where each repetition consisted of three Petri dishes (experimental units). All evaluations were performed in triplicate. The PIRG and halo inhibition data were analyzed with analysis of variance (ANOVA) using the Statistical Analysis System version 9.0 (SAS, 2002). Tukey test (p=0.05) was used for means comparison.
Results and Discussion
Ten bacterial isolates were obtained, mainly corresponding to the Bacillus genus according to their morphological characters. The morphological identity was verified by means of sequence comparison from the obtained in this research with those recorded in the NCBI GenBank and RDP (Table 2). The municipality of Tepic presented the highest frequency of isolates of antagonistic bacteria, a strain belonging to Burkholderia cepacia, one to Bacillus sp., three to B. amyloliquefaciens and three to B. subtilis, while in the municipality of Xalisco two isolates were obtained, one of B. amyloliquefaciens and another of B. subtilis.
Tabla 2 Molecularly identified antagonistic bacteria from the rhizosphere of commercial avocado orchards in the municipalities of Tepic and Xalisco, Nayarit, Mexico.
| Origen/Locality | Key of the isolated | Molecular identification |
|---|---|---|
| Ahuacate | Bc-02 | Burkholderia cepacia |
| Camichin II | Ba-12 | Bacillus amyloliquefaciens |
| Camichin III | Bsp-03 | Bacillus sp. |
| Carbonera | Ba-04 | Bacillus amyloliquefaciens |
| Comunidad Indígena | Bs-06 | Bacillus subtilis |
| Fortuna | Bs-11 | Bacillus subtilis |
| Guayabitos | Bs-09 | Bacillus subtilis |
| Noria | Bs-07 | Bacillus subtilis |
| Noria | Ba-05 | Bacillus amyloliquefaciens |
| Yerba | Ba-08 | Bacillus amyloliquefaciens |
Antagonistic activity of bacterial isolates against phytopathogens.
According to the in vitro confrontations, it was determined that the 10 bacterial isolates showed antagonistic activity against Phytophthora cinnamomi, Fusarium oxysporum and Colletotrichum sp. (Figure 1). The Bc-02 and Ba-05 isolates identified as B. cepacia and B. amyloliquefaciens, respectively, showed the highest inhibition capacity of 70.24 % and 61.08 % on P. cinnamomi, while the Bm-10 isolate identified as B. amyloliquefaciens showed the lowest PIRG (42.25 %) against P. cinnamomi (Figure 1A). Additionally, a reduction of 9.1 mm/day in the mycelial growth rate of P. cinnamomi with the Bc-02 isolate was observed.
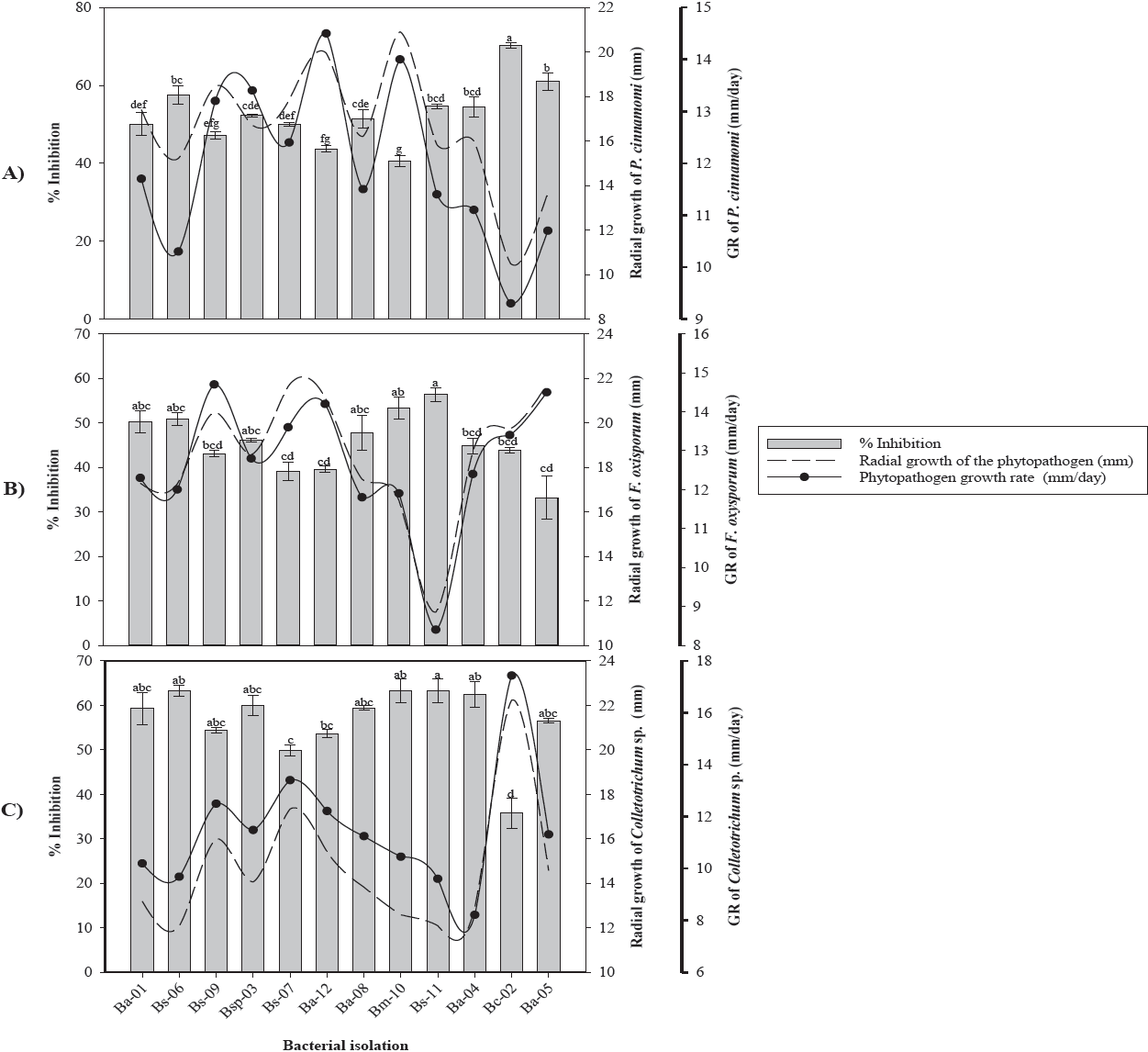
Figure 1 Antifungal effect of bacterial strains on radial growth, growth rate and percentage of mycelial inhibition of A) Phytophthora cinnamomi, B) Fusarium oxysporum and C) Colletotrichum sp. * GR= Growth rate. Values with the same letter are statistically equal according to the Tukey test (p=0.05).
In vitro assays of Ba-05 and Bs-11 isolates against F. oxysporum showed inhibitions of 33 % to 56.33 % with reductions in their growth rate of 7.33 mm/day, respectively (Figure 1B). The Bs-11 and Bs-06 isolates identified as B. subtilis showed inhibitions greater than 63.24 % on Colletotrichum sp., while the B. cepacia isolate (Bc-06) showed the lowest inhibition (35.72 %) compared to the control (Figure 1C). In this fungus, a reduction in the mycelial growth rate of 10.47 mm/day was observed when confronted with the isolated Bs-11 of B. subtilis.
Of the 10 bacterial isolates obtained from the rhizosphere of avocado trees, 90 % belonged to the Bacillus genus and 10 % to the Burkholderia genus. These results coincide with those obtained by Pozo et al. (2007) who isolated bacteria from different regions of Havana, Cuba, with a large number of strains corresponding to the Bacillus spp genus. Garbeva et al. (2003) in studies of the microbiota of agricultural soils determined that approximately 95 % of the DNA belonged to the genera Bacillus and Paenibacillus. The Bc-02 isolate of B. cepacia showed the highest growth inhibition (70.24 %) on P. cinnamomi, followed by Ba-05 and Bs-06 isolates belonging to B. amyloliquefaciens and B. subtilis, with growth inhibitions of 61.08 % and 57.54 %, respectively. These results coincide with Ezziyyani et al. (2004), who reported B. cepacia with a Phytophthora capsici inhibition of 81 % at 96 h and at 8 d was overgrowth by the bacteria. This isolate has the potential to be used as a biological control agent for phytopathogens due to its high inhibitory capacity attributed to the production of secondary metabolites such as pyrrolnitrine, altericidine, cepacin, among other volatile and non-volatile compounds (Kraus & Loper, 1995; Rosales et al., 1995; El-Banna et al., 1998; De La Fuente et al., 2001; Quan et al., 2006).
Growth inhibition of F. oxysporum and Colletotrichum sp., induced by Bacillus spp. was significantly different. These differences could be due to the ability of each isolate to produce antimicrobial compounds such as bacillomycin, mycosubtilin, fungimycin and zwittermicin that inhibit the growth of various microorganisms including fungi (Pal & Gardener, 2006; Madhaiyan et al., 2010; Zhang et al., 2012). In this regard, Edwards et al. (1994) mention that antibiosis is the most common mode of action in the Bacillus genus. Fusarium oxysporum and Colletotrichum sp. showed growth inhibitions of 56.33 % and 64.43 %, induced by the Bs-11 isolate of B. subtilis. Corrales et al. (2011) reported this species with an in vitro inhibition of 71.71 % on Fusarium spp. Kim et al. (2010) reported the production of fractions of iturin A and fengicin by a strain of B. subtilis when confronted against Colletotrichum gloeosporioides.
Iturin group antibiotics are secreted by most strains of Bacillus spp. and inhibit mycelial growth and sporulation of the fungus by altering membrane permeability and fungal cell lipid composition (Romero et al., 2007; Li et al., 2009).
The inhibitions showed by Bacillus methylotrophicus (Bm-10) on Fusarium sp. (53.32 %) and Colletotrichum spp. (63.16 %), were similar to those reported by Rios et al. (2016). In this regard, Madhaiyan et al. (2010) and Zhang et al. (2012), showed that B. methylotrophicus has antifungal ability against a great diversity of phytopathogenic fungi.
Bacillus methylotrophicus (Bm-10) and B. subtilis (Bs-11) showed the highest antifungal capacity against the three fungi, which could be due to the production of antibiotics and/or volatile compounds such as hydrogen cyanide, which has been reported as one of the responsible for the inhibition of the growth of phytopathogenic fungi, besides of the harmful effects on the in vitro growth of several phytopathogens by affecting the activity of specific fungal enzymes (Wheatley, 2002; Duffy et al., 2003; Correa et al., 2009; Zhang et al., 2012). In this regard, Souto et al. (2004) reported that the antifungal ability of B. amyloliquefaciens when confronted in vitro is due to the production and release of peptides and lipopeptides into the culture medium, such as fungicine, iturin, bacillomycin, among others. Determine the antagonistic capacity of bacterial strains on the different phytopathogens is of great interest since some strains evaluated in our study showed greater in vitro antifungal capacity against P. cinnamomi and less effective when confronted with F. oxysporum (Figure 1B). These results show the variability between isolates due to the specificity of antibiotics produced by bacteria (Schippers et al., 1987). Besides the fungal defense mechanisms.
Conclusion
In rhizospheric soils of avocado orchards in Nayarit, diversity of bacterial strains exists, since we identified species of Bacillus subtilis, Bacillus amyloliquefaciens, Burkholderia cepacia and Bacillus sp. with an in vitro antagonistic capacity against Phytophthora cinnamomi, Fusarium oxysporum and Colletotrichum sp. Some of these bacteria could be potential candidates to be included in integrated management schemes of the three fungi of agricultural interest studied.











 texto en
texto en 


