Introduction
The fungi Fusarium graminearum, F. verticillioides, F. proliferatum and F. subglutinans are considered the most common pathogens of maize (Leslie & Summerell, 2006); the phytopathogen F. verticillioides is a species of greatest relevance and interest in maize (Duncan & Howard, 2010), it has a wide distribution and is endemic in all maize-producing regions of the world (Blacutt et al., 2018), greatly limits its production causing considerable yield losses; which is at a global level around 7-17 % while in Mexico it oscillates around 7.5-38 % (González et al., 2007). Likewise, it causes changes in the nutritional content, reducing the content of lipids, proteins and carbohydrates, in addition to altering the organoleptic properties (Chavarri et al., 2017) besides of contaminating the grains with mycotoxins (Giorni et al., 2019); this fungus is known to be found more frequently on the cob in corn-producing areas (Briones-Reyes et al., 2015), affecting all phenological stages of the maize and different parts of it by inducing pre- and post-harvest diseases that cause a reduction of yield and affect seed quality (Schulthess et al., 2002).
The Fusarium genus produces three groups of toxins which are: zearalenone, trichothecenes, and fumonisins which are mainly found in maize, and are toxic to humans and animals that consume them (Agrios, 2005). The fungus F. verticillioides in its infection produces fumonisins; it has been studied that exposure to fumonisin B1 (FB1) from maize produces leukoencephalomalacia (LEM) in horses as well as pulmonary edema in pigs. FB1 also produces toxic effects on the central nervous system, liver, pancreas, kidneys and lungs of several animal species (FAO, 2020), FB1 and FB2 have also been associated with esophageal carcinoma in rats and moniliformin (MON) with myocardial damage (Bertuzzi et al., 2020). For many years, the control of fungal diseases has largely depended to a large degree on chemical control (Kumar & Ashraf, 2017). Nevertheless, the use of chemical molecules represents a threat to human health and contributes to an increase of environmental pollution (Abdel-Monahim et al., 2011), and has given pace to the appearance of resistant strains that lead to a higher incidence of fungal diseases (Hernández et al., 2014). To reduce this problem, it is necessary to search for and adopt new accessible and simply applicable strategies that are innocuous to humans and animals (Naeini et al., 2010).
Biological control is an excellent alternative as an option to the use of chemical fungicides for the management of diseases in organic and conventional production systems, with the least possible negative effect on the environment (Nagarajkumar et al., 2004). Trichoderma species are commonly the most applied fungi as biological control agents in agriculture (Diánez et al., 2016), the ability of Trichoderma to control plant diseases is based on the activation of one or multiple control mechanisms (Muhammad et al., 2019), these mechanisms are based by mycoparasitism (Elamathi et al., 2018), production of antibiotics and hydrolytic enzymes (Vinale et al., 2008), competition for space and nutrients (Oszust et al., 2020), the induction of systemic resistance in the plant (Contreras-Cornejo et al., 2014), and the production of secondary metabolites (Zeilinger et al., 2016). Therefore, this work aimed to evaluate the effectiveness of three Trichoderma strains for the control of ear rot in four corn genotypes.
Material and Methods
Trichoderma Strains
Trichoderma species were obtained from the fungal culture collection of the Phytopathology Laboratory of the Parasitology Department of the “Universidad Autónoma Agraria Antonio Narro” in Mexico, isolated from Mexican agricultural soils. The cultures were reactivated with potato dextrose agar (PDA) medium and massively increased in rice seeds according to the methodology suggested by Michel-Aceves et al. (2008), the species used were, T. harzianum, T. asperellum and T. longibrachiatum.
Fusarium verticillioides isolation
At the end of the spring-summer cycle, specifically at the time of harvest, a sampling of the zone established as the experiment was done and 110 days after sowing stems and cobs of symptomatic and asymptomatic native maize were collected to obtain the phytopathogen. The stems and cobs were transferred to the Parasitology Department and fractions were inoculated in Malachite Green Agar culture medium (Leslie & Summerell, 2006). This was done following the method described by Agrios (2005).
Morphological characterization of Fusarium verticillioides as a causal agent of corn ear rot
Strains were phenotypically characterized according to Leslie & Summerell (2006), purification was carried out from monoconidial cultures, isolates were kept at 4 °C until use (Duarte et al., 2016).
Molecular characterization of F. verticillioides
The pathogen was cultured in PDA medium for 10 days. DNA extraction was carried out using the methodology of Nicholson et al. (2001). DNA integrity and quality of the extracted sample were checked on 1 % agarose gel by horizontal electrophoresis running at 100 V for 40 min. The Internal Transcribed Spacer 1 (ITS1) and ITS4 were amplified using specific primers for ITS1 (5’TCC GTA GGT GAA CCT GCG G3’) and ITS4 (5’TCC GCT TGA TAT GC3’). The final reaction volume was 20μL, which contained 13.58 of MQ water, 2.0 μL of MgCl buffer (10X), 0.32 μL of MgCl2 (25 mM), 0.4 μL of dNTP´s (10 mM), 0.5 μL of the primers for ITS1 and ITS4 at a 10 μM concentration, 0.5 μL of DMSO, 0.2 μL of DNA Taq-polymerase 1 U and 1 μL of DNA (40 ng/ μL) The amplification reaction conditions were: 1 cycle of 94 °C 3min, followed by 35 cycles at 94 °C 45s, 53 °C for 45s and 72 °C 1 min; ending with a polymerization cycle of 72 °C for 7 min. The amplified bands were observed in 1 % agarose gel at 90 V for 60 min.
PCR products were sequenced by Macrogen laboratory (Rockville, USA). The obtained sequences were compared with those registered at the GenBank database of the National Center for Biotechnology Information (NCBI), the BLAST tool (Basic Local Alignment Search Tool) was used for highly similar sequences.
Trichoderma spp. conidia suspension
The conidia suspension of Trichoderma species was obtained from 25-day-old cultures grown in rice grains at 26 °C, the conidia were recovered with sterile distilled water, filtered with gauze to separate mycelium and conidia (Gato, 2010), the filtration was adjusted to the concentration of 1x109 conidia/mL in a Neubauer chamber (Neubauer improved bright-line, Lo-Laboroptik).
Genotypes
Native and hybrid maize varieties with adaptation to tropical zones were used, the H-520 F1 and Mestizo Diamante hybrids were provided by Grupo Hernández Montiel y Asociados S.P.R de R.L., the native varieties were acquired from local producers in the study area and the UAAAN-ISP-173 material was provided by the Banco Nacional de Germoplasma de Maíces de México of the Universidad Autónoma Agraria Antonio Narro, and was used for being known to be tolerant to Fusarium verticillioides invasion.
Biocontrol activity in plant
The sowing of the maize plants was done manually in the fall-winter cycle under rainwater irrigation and natural F. verticillioides infection, in furrows of 5 m in length with 0.9 m of distance between furrows and 0.4 m between plants in the region of the Huasteca of Veracruz, in the Chapopote II ejido localized at the coordinates 21°10´13.68´´N and 98°13´36.09´´ W. A randomized block design with factorial arrangement A x B x C was used, with four repetitions per treatment (5 furrows/ repetition) and an absolute witness were used as control. Twenty-five seeds were sown in each furrow inoculated and uninoculated with Trichoderma spp. and two seeds were deposited in each furrow. The population density was approximately 55,555 plants/hectare.
The Trichoderma species were inoculated by three different methods; seed inoculation treatment (TS), foliar spray (TA) and the two previous methods in combination (TS+TA).
The dose applied in the seed inoculation treatment was equivalent to 10 g/kg of seed (Chandra et al., 2008). To do so, the seed was hydrated in water for 12 h, and the sap of Aloe vera was used as a natural adherent.
Inoculation by spraying was carried out when more than 50 % of the female flowers had emerged, in the R1 stage applications was directed towards the stigma on three occasions, at a concentration of 1x109 conidia/mL, with repetitions every third day, and suspended when the population had become uniform around 95 % of flowering.
Biocontrol effect
Effect on the incidence of ear rot
At 150 days after sowing, 20 plants were selected at random from the three central furrows of each replicate, their cobs were harvested for the evaluation of rot incidence, the cobs that showed rot signals in the plots of each treatment were counted, and the results were expressed as a percentage.
Determination of ear rot severity
Twenty randomly selected cobs were harvested from the three central furrows. Severity was determined by the scale of Reid et al. (1996). Table 1.
Statistical analysis
The incidence was processed by analysis of variance with a factorial array of three factors (A x B x C), with three levels in factor A, four levels in B and four in C. The methods were placed in factor A, the used genotypes in factor B, and factor C was composed by the strains of Trichoderma used. The software SAS 9.0 for windows was used and the measures were separated by a Tukey test with a significance of p = 0.05.
Disease severity data were adjusted to a weighted mean using Friedman’s test, with SAS version 9.0 for Windows.
Results and Discussion
Morphological characterization of Fusarium verticillioides as a causal agent of ear rot
After 12 days of incubation of the samples of the phytopathogenic fungus an extensive cottony mycelium was observed in the culture medium, frequently with dashes of pink, purple or yellow in the medium. Abundant single and stranded microconidia were found, usually, single-cell, double-cell, oval and club-shaped hyaline and were generally flattened at each end. Macroconidia, with thin walls and their shape from canoe-shaped to almost straight; with 3-7 septa and the basal cell in the shape of a foot. In the absence of chlamydospores in the mycelium, F. verticillioides was identified as the cause of ear rot according to phenotypic characteristics described by different authors (Warham et al., 1996; Barnett & Hunter, 1998; Leslie & Summerell, 2006).
Molecular characterization of F. verticillioides
According to the sequences compared in GenBank, and access number AB587010, the fungus F. verticillioides was confirmed to have 100 % homology with the sequences of F. verticillioides strain CBS 576.78.
Effect of biocontrol on the incidence of ear rot
The effect of biocontrol evaluated at 150 days after sow, showed that the three application methods (p ≤ 0.0002) and the three species of Trichoderma spp. (p ≤ 0.0001) significantly reduced the incidence of ear rot caused by F. verticillioides. The incidence of rot oscillated around 47 to 79 % in the treatments, being TS+TA the most effective, increasing plant health in 53 % as compared to the witness condition which presented the highest incidence (Figure 1). Similar results were previously reported by Chandra et al. (2008) who pointed out that with the application of antagonistic microorganisms such as T. harzianum, the incidence was reduced by more than 50 %. Another study by Ferrigo et al. (2014) indicated that T. harzianum T22 reduced the colonization of F. verticillioides by 58 % through seed treatment, which makes the use of Trichoderma promising as way to reduce F. verticillioides damage, as observed in this research.
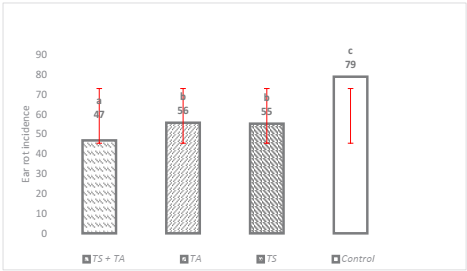
The means with the same letter are not significantly different according to the Tukey test, tested at p = 0.05. Bars are the standard deviation of the mean.
Figure 1 Effect of Trichoderma spp. on the incidence of ear rot with three different treatments.
Infection of maize by F. verticillioides can occur through several routes, the most commonly reported method for infection of the grain is through airborne conidia that infect the stigmas, and thereafter the invasion of the stigmas, the fungus infects the seeds (Sartori et al., 2015). Systemic infection can begin with conidia or mycelium of the fungus being transported within the seeds or on the surface of the seeds. The fungus develops inside the young plant, passing from the roots to the stem and finally to the cob and grains (Duncan & Howard, 2010). The results obtained in the present research make evident that by treating the different access points of F. verticillioides with different species of Trichoderma in different maize genotypes, the incidence and severity of the disease can be reduced at different levels of biocontrol, with the best result being found with seed inoculation and foliar spraying in combination.
On the other hand, the three strains of Trichoderma significantly reduced the incidence of ear rot (p ≤ 0.0001) at different levels of biocontrol, however, such effect was especially notorious in the strain of T. harzianum, which presented the lowest level of incidence with respect to the control. It is important to mention that of the three tested strains, T. longibrachiatum was the one that presented the highest incidence, followed by T. asperellum and T. harzianum as shown in Figure 2. No statistical differences were observed between T. asperellum and T. harzianum, nevertheless, the biocontrol effect was statistically superior with respect to the control. This biocontrol effect was also documented by Cavaglieri et al. (2005) due to the application of Bacillus subtilis in maize plants under greenhouse conditions where the reduction of rhizoplane and endorhizosphere colonization by F. verticillioides was observed.

The means with the same letter are not significantly different according to the Tukey test, tested at p = 0.05. Bars are the standard deviation of the mean.
Figure. 2 Effect of Trichoderma spp. on the incidence of ear rot with three different strains.
The genus Trichoderma comprises a large number of species that can act as biological control agents, whose antagonistic properties are based on the activation of multiple mechanisms of action (Mukherjee et al., 2014). For this study, biocontrol could be observed in the three strains of Trichodema with different levels of effectiveness. This was probably because the different strains exerted more than one mechanism of action to suppress the growth and development of F. verticillioides thus diminishing the incidence and severity of the disease. Trichoderma species act against fungal phytopathogens either indirectly, by competing for nutrients and space, modifying environmental conditions, promoting plant growth or systemic plant defense and through antibiosis, or directly, by mechanisms such as mycoparasitism (Gwa & Nwankiti, 2017).
These indirect and direct mechanisms can act in a coordinated way and their importance in the biocontrol process depends on the strain of Trichoderma, the antagonized fungus, the plant species and the environmental conditions, including the availability of nutrients, pH, temperature, among others (Filizola et al., 2019). The activation of each mechanism implies the production of specific compounds and metabolites, such as plant growth factors, hydrolytic enzymes, siderophores, antibiotics, and nitrogen (Rubio et al., 2014). The results presented are statistically superior to the control and it can be observed that Trichoderma strains exerted different levels of control and that in each technique evaluated, the strains may have coordinated direct and indirect mechanisms as part of the biocontrol.
Effect on disease severity
For severity, relatively low levels were found in all three treatments protocols, with TS+TA having the lowest severity compared to the rest of the treatments; all three treatments were placed in class 2 in the scale of Reid et al. (1996) with respect to the control, which had damage levels of 10.89 % and was placed in class 3 of the same scale in relation to the weighted average. The Friedman test confirmed that at least two treatments are different at the significance level of p < 0.05. The effectiveness of the treatments was determined by means of the Abbott formula (1925) and the following results were obtained (Table 2). In all the treatments, the effectiveness was above 70 %, and it was also observed that in all the treatments where the Trichoderma strains were applied, the flowering was at least one week ahead as compared to control. In other studies, conducted by Chandra et al. (2009) showed that seed treatment and spray treatment of a pure culture of Pseudomonas fluorescens reduced the incidence of ear rot by 83 %, with powdered formulations of talc, corn starch and wheat bran reduced ear rot by 81, 79 and 77 %, respectively.
Table 2 Technical efficiency of treatments with three strains of Trichoderma spp. in four maize genotypes on the severity of ear rot. (Abbott, 1925).
| Genotypes | Treatments | Strains | ||
|---|---|---|---|---|
| T. longibrachiatum | T. asperellum | T. harzianum | ||
| H-520 | TS | 81.04 ± 0.00a | 75.07 ± 5.61a | 79.34 ± 2.73a |
| H-520 | TA | 79.24 ± 2.45a | 79.24 ± 3.68a | 77.73 ± 3.87a |
| H-520 | TS+TA | 78.86 ± 2.49a | 79.24 ± 3.68a | 77.91 ± 3.61a |
| MESTIZO | TS | 78.67 ± 3.50a | 77.98 ± 3.25a | 73.58 ± 7.34a |
| MESTIZO | TA | 76.91 ± 2.14a | 73.48 ± 0.00a | 78.67 ± 1.05a |
| MESTIZO | TS+TA | 76.91 ± 2.11a | 80.43 ± 0.00a | 75.73 ± 3.36a |
| CRIOLLO | TS | 74.64 ± 1.06a | 74.82 ± 2.91a | 76.98 ± 3.65a |
| CRIOLLO | TA | 78.60 ± 1.01a | 76.80 ± 2.03a | 79.77 ± 3.35a |
| CRIOLLO | TS+TA | 76.44 ± 1.23a | 80.31 ± 2.03a | 82.01 ± 0.00a |
| UAAAN-ISP-173 | TS | 80.99 ± 2.83a | 77.91 ± 3.61a | 80.48 ± 2.75a |
| UAAAN-ISP-173 | TA | 77.74 ± 1.51a | 75.86 ± 0.54a | 80.74 ± 0.00a |
| UAAAN-ISP-173 | TS+TA | 76.80 ± 1.51a | 77.91 ± 0.56a | 76.80 ± 1.74a |
TS (seed treatment), TA (spray treatment) and TS + TA (seed treatment plus spray treatment). Means with the same letter in the same column are not significantly different according to the Tukey test, tested at p = 0.05 ± are mean standard deviation.
Conclusion
F. verticillioides was identified morphologically as the cause of ear rot in maize genotypes of the state of Veracruz. The strain T. harzianum presented the highest significant effectivity on the reduction of incidence and severity of the disease. The most promising method of application of Trichoderma spp. was treatment of inoculation of the seed combined with foliar aspersion, for which by treating the routes of entry of the pathogen can reduce the frequency of F. verticilloides, due to that direct or indirect mechanisms of the strains of Trichoderma can act in a coordinated manner in the process of biocontrol.











 text in
text in 



